404 Not Found
Additional ordering information
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
Storage, safety and handling
| Intended use | Research Use Only (RUO) |
Example protocol
AT A GLANCE
Protocol Summary
- Prepare samples in microplate wells
- Remove liquid from samples in the plate
- Add AF350-Phalloidin solution (100 μL/well)
- Stain the cells at room temperature for 20 to 90 minutes
- Wash the cells
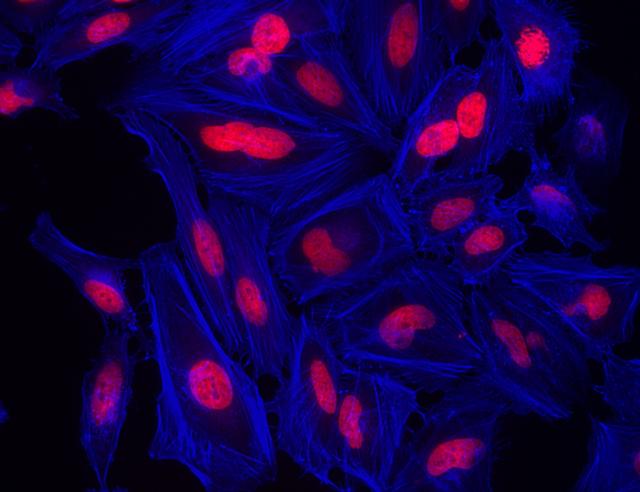
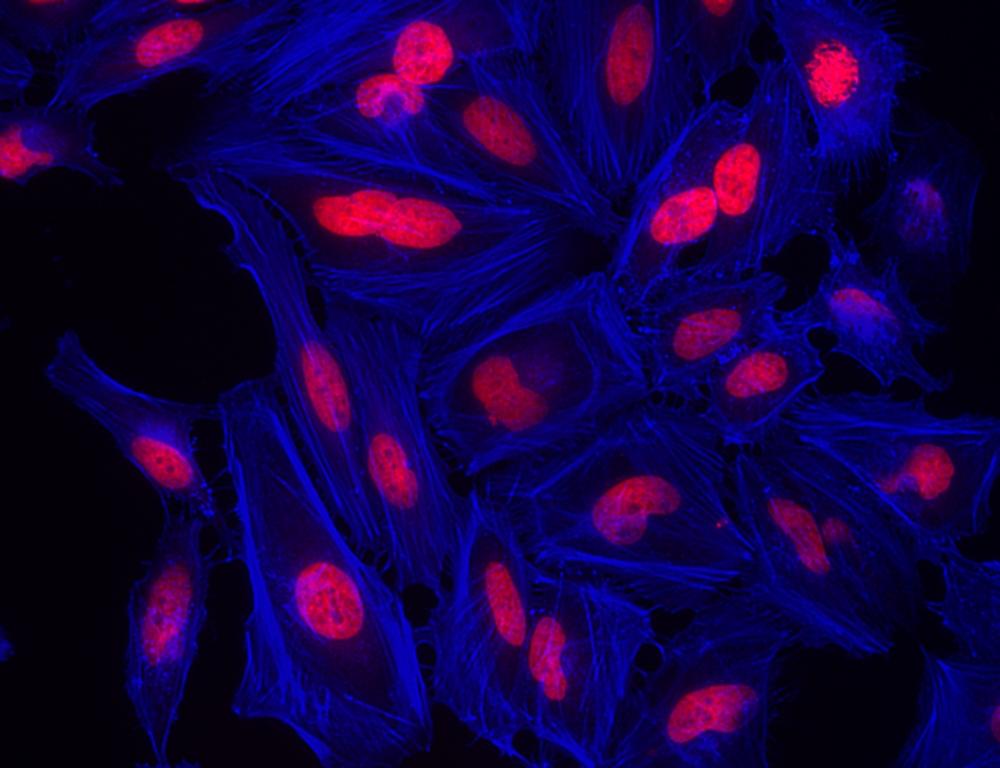
- Examine the specimen under microscope with DAPI filter
Storage and Handling Conditions
The solution should be stable for at least 6 months if store at -20 °C. Protect the fluorescent conjugates from light, and avoid freeze/thaw cycles.Note Phalloidin is toxic, although the amount of toxin present in a vial could be lethal only to a mosquito (LD50 of phalloidin = 2 mg/kg), it should be handled with care.
PREPARATION OF WORKING SOLUTION
AF350-Phalloidin working solution
Add 1 µL of AF350-Phalloidin solution to 1 mL of PBS with 1% BSA.Note The stock solution of phalloidin conjugate should be aliquoted and stored at -20 °C. protected from light.
Note Different cell types might be stained differently. The concentration of phalloidin conjugate working solution should be prepared accordingly.
SAMPLE EXPERIMENTAL PROTOCOL
Stain the cells
- Perform formaldehyde fixation. Incubate cells with 3.0–4.0 % formaldehyde in PBS at room temperature for 10–30 minutes.
Note Avoid any methanol containing fixatives since methanol can disrupt actin during the fixation process. The preferred fixative is methanol-free formaldehyde. - Rinse the fixed cells 2–3 times in PBS.
- Optional: Add 0.1% Triton X-100 in PBS into fixed cells for 3 to 5 minutes to increase permeability. Rinse the cells 2–3 times in PBS.
- Add 100 μL/well (96-well plate) of AF350-Phalloidin working solution into the fixed cells, and stain the cells at room temperature for 20 to 90 minutes.
- Rinse cells gently with PBS 2 to 3 times to remove excess phalloidin conjugate before plating, sealing and imaging under microscope with DAPI filter set.
Images
Application notes
A New Protein Crosslinking Method for Labeling and Modifying Antibodies
A New Robust No-Wash FLIPR Calcium Assay Kit for Screening GPCR and Calcium Channel Targets
A Novel NO Wash Probeniceid-Free Calcium Assay for Functional Analysis of GPCR and Calcium Channel Targets
Buccutite™ Bioconjugation Technology
Fluorescent Oligonucleotide Labeling Reagents
A New Robust No-Wash FLIPR Calcium Assay Kit for Screening GPCR and Calcium Channel Targets
A Novel NO Wash Probeniceid-Free Calcium Assay for Functional Analysis of GPCR and Calcium Channel Targets
Buccutite™ Bioconjugation Technology
Fluorescent Oligonucleotide Labeling Reagents