Amplite® Fluorimetric Myeloperoxidase Assay Kit *Red Fluorescence*
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
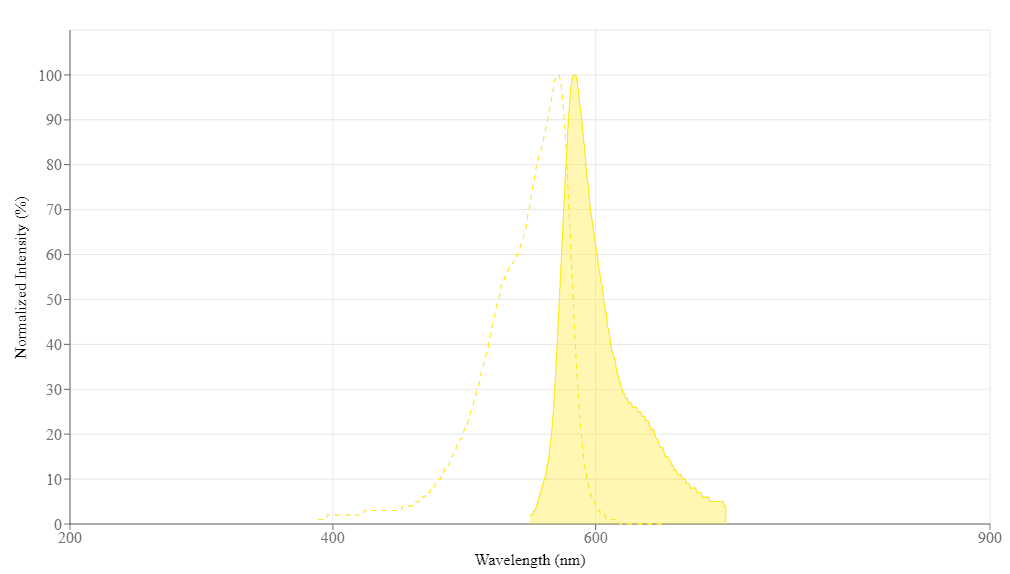
| Excitation (nm) | 571 |
| Emission (nm) | 584 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
| Overview |
Excitation (nm) 571 | Emission (nm) 584 |
Platform
Fluorescence microplate reader
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black |
Components
Example protocol
AT A GLANCE
Protocol summary
- MPO standards or test samples (50 µL)
- Add MPO working solution (50 µL)
- Incubate at room temperature for 30 - 60 min
- Read fluorescence intensity at Ex/Em = 540/590 nm (cut off 570 nm)
Important notes
Thaw all the kit components to room temperature before starting the experiment.
PREPARATION OF STOCK SOLUTION
1. Amplite™ Red stock solution (250X):
Add 40 µL of DMSO (Component E) into the vial of Amplite™ Red substrate (Component A). The stock solution should be used promptly. Note: The Amplite™ Red substrate is unstable in the presence of thiols such as dithiothreitol (DTT) and 2-mercaptoethanol. The final concentration of DTT or 2-mercaptoethanol in the reaction should be no higher than 10 µM. The Amplite™ Red substrate is also unstable at high pH (>8.5). Therefore, the reaction should be performed at pH 7 – 8. The provided assay buffer, pH 7.4, is recommended.
2. H2O2 stock solution (500X, 10 mM):
Add 10 µL of 3% H2O2 (0.88M, Component C) into 870 µL of Assay Buffer (Component B). Note: The diluted H2O2 solution is not stable. The unused portion should be discarded.
3. Myeloperoxidase (MPO) standard solution (200 mU/mL):
Add 50 µL of Assay Buffer (Component B) into the vial of Myeloperoxidase Standard (Component D). Note: One vial contains approximately 5 - 10 mU myeloperoxidase.
PREPARATION OF STANDARD SOLUTION
For convenience, use the Serial Dilution Planner: https://www.aatbio.com/tools/serial-dilution/11301
Add 20 µL of 200 mU/mL MPO standard solution into 380 µL of Assay Buffer (Component B) to get 10 mU/mL MPO standard solution (MPO7). Take 10 mU/mL MPO standard solution to perform 1:3 serial dilutions to get remaining serially diluted MPO standards (MPO6 - MPO1).
PREPARATION OF WORKING SOLUTION
Add 20 μL of Amplite™ Red Stock Solution (250X) and 10 μL of H2O2 (500X) into 5 mL of Assay Buffer (Component B) to make a total volume of 5.03 mL MPO working solution. Protect from light.
SAMPLE EXPERIMENTAL PROTOCOL
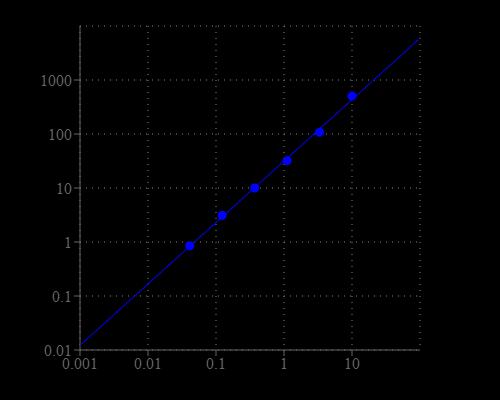
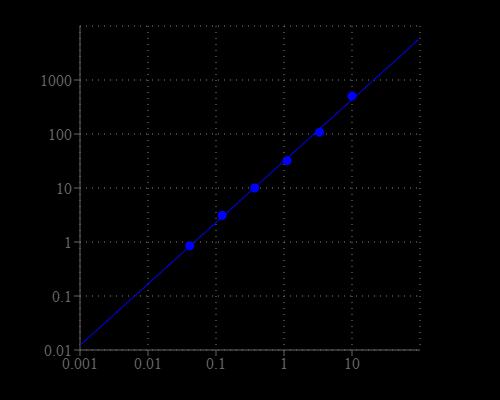
Table 1. Layout of MPO standards and test samples in a 96-well solid black microplate. MPO= myeloperoxidase standards (MPO1 - MPO7, 0.01 to 10 mU/mL); BL=blank control; TS = test samples.
| BL | BL | TS | TS |
| MPO1 | MPO1 | ... | ... |
| MPO2 | MPO2 | ... | ... |
| MPO3 | MPO3 | ||
| MPO4 | MPO4 | ||
| MPO5 | MPO5 | ||
| MPO6 | MPO6 | ||
| MPO7 | MPO7 |
Table 2. Reagent composition for each well. Note that high concentration of MPO may cause reduced fluorescence signal due to the over oxidation of Amplite™ Red substrate (to a non-fluorescent product).
| Well | Volume | Reagent |
| MPO1 - MPO7 | 50 µL | Serial Dilution (0.01 to 10 mU/mL) |
| BL | 50 µL | Assay Buffer (Component B) |
| TS | 50 µL | test sample |
- Prepare myeloperoxidase standards (MPO), blank controls (BL), and test samples (TS) according to the layout provided in Tables 1 and 2. For a 384-well plate, use 25 µL of reagent per well instead of 50 µL.
- Add 50 µL of MPO working solution to each well of myeloperoxidase standard, blank control, and test samples to make the total MPO assay volume of 100 µL/well. For a 384-well plate, add 25 µL of MPO working solution into each well instead, for a total volume of 50 µL/well.
- Incubate the reaction for 30 to 60 minutes at room temperature, protected from light.
- Monitor the fluorescence intensity with a fluorescence plate reader at Excitation = 530 - 570 nm, Emission = 590 - 600 nm (optimal Ex/Em = 540/590 nm, cut off = 570 nm). Note: The contents of the plate can also be transferred to a white clear bottom plate and read by an absorbance microplate reader at the wavelength of 576 ± 5 nm. The absorption detection has lower sensitivity compared to that of the fluorescence reading.
Images
Citations
Authors: Akentieva, Natalia and Sanina, Natalia and Gizatullin, Artur and Shkondina, Natalia and Andreeva, Anna and Shram, Stanislav and Aldoshin, Sergei
Journal: (2022)
References
Authors: Tan S, Wang G, Peng M, Zhang X, Shen G, Jiang J, Chen F.
Journal: Clin Chim Acta (2009): 216
Authors: Zelzer S, Khoschsorur G, Stettin M, Weihrauch G, Truschnig-Wilders M.
Journal: Clin Chim Acta (2009): 62
Authors: Grulke S, Franck T, Gangl M, Peters F, Salciccia A, Deby-Dupont G, Serteyn D.
Journal: Can J Vet Res (2008): 37
Authors: Fietz S, Bondzio A, Moschos A, Hertsch B, Einspanier R.
Journal: Res Vet Sci (2008): 347
Authors: Sakamoto W, Fujii Y, Kanehira T, Asano K, Izumi H.
Journal: Clin Biochem (2008): 584
Authors: Franck T, Grulke S, Deby-Dupont G, Deby C, Duvivier H, Peters F, Serteyn D.
Journal: J Vet Diagn Invest (2005): 412
Authors: Dypbukt JM, Bishop C, Brooks WM, Thong B, Eriksson H, Kettle AJ.
Journal: Free Radic Biol Med (2005): 1468
Authors: Kapuscinska R, Wysocka J, Niczyporuk W, Ratomski K.
Journal: Wiad Lek (2004): 599
Authors: Yu F, Zhao MH, Zhang YK, Wang HY.
Journal: Zhonghua Nei Ke Za Zhi (2003): 27
Authors: Haqqani AS, S and hu JK, Birnboim HC.
Journal: Anal Biochem (1999): 126
Application notes
Design of potent inhibitors of acetylcholinesterase using morin as the starting compound
Acetylcholinesterase Inhibitory Activity of Pigment Echinochrome A
Induction of Neurite Outgrowth in PC12 Cells
Induction of Neuritogenesis in PC12 Cells by a Pulsed Electromagnetic Field
FAQ
How should I reconstitute an NADPH standard?
Will Amplite® Fluorimetric NAD/NADH Ratio Assay Kit *Red Fluorescence* work with NADP/NADPH? Can this kit measure NADP+ and NADPH?
What is the concentration of calcium inside cells?
What assay kits measure NADP/NADPH from cell samples?