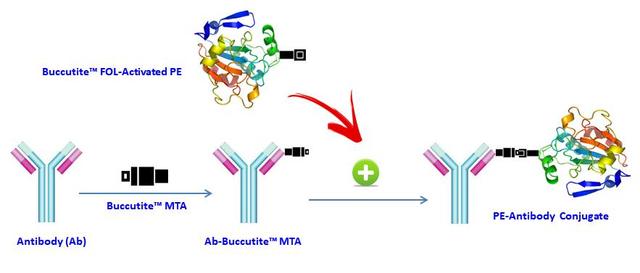
Buccutite™ Rapid PE-Cy5.5 Tandem Antibody Labeling Kit *Microscale Optimized for Labeling 100 ug Antibody Per Reaction*
Ordering information
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
Additional ordering information
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
Spectral properties
| Extinction coefficient (cm -1 M -1) | 1960000 |
| Excitation (nm) | 565 |
| Emission (nm) | 671 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Alternative formats
| Buccutite™ Rapid PE-Cy5.5 Tandem Antibody Labeling Kit *Microscale Optimized for Labeling 25 ug Antibody Per Reaction* |
Related products
| Overview |
See also: Antibody and Protein Labeling, Protein to Protein Conjugation, Buccutite™ Crosslinkers and Kits, Flow Cytometry Reagents, PE and APC, Spectral Flow Cytometry
Extinction coefficient (cm -1 M -1) 1960000 | Excitation (nm) 565 | Emission (nm) 671 |
PE-Cy5.5 is a popular color used in flow cytometry. Its primary absorption peak is at 565 nm with emission peak at~700 nm. The filter sets of 682/33 nm and 695/40 nm are recommended for this tandem color. AAT Bioquest offers this Buccutite™ rapid labeling kit to facilitate the PE-Cy5.5 tandem conjugations to antibodies and other proteins such as streptavidin and other secondary reagents. Buccutite™ PE-Cy5.5 Conjugation Kit provides a robust and convenient method to conjugate your antibodies with PE. The kit includes an activated PE and reaction buffer. The conjugated antibody can be used in flow cytometry, WB, ELISA and IHC applications. This kit is sufficient for 2 labeling reactions, each up to 100 ug of antibody. Considering the large size of PE (240 kDa), the amount of antibody used in a labeling reaction must always be less than the amount of RPE. The best ratio for any new antibody reagent must be determined by experimentation but 50-60 ug of IgG antibody for every 100 ug of RPE usually gives optimal results. Our kit provides preactivated PE-Cy5.5 to facilitate the PE-Cy5.5 tandem conjugations to antibodies and other proteins such as streptavidin and other secondary reagents. Our preactivated PE-Cy5.5 tandem is ready to conjugate, giving much higher yield than the conventionally tedious SMCC-based conjugation chemistry. In addition, our preactivated PE-Cy5.5 tandem is conjugated to a protein via its amino group that is abundant in proteins while SMCC chemistry targets the thiol group that has to be regenerated by the reduction of antibodies.
Components
Example protocol
AT A GLANCE
Protocol Summary
- Add 5 µl Reaction Buffer (Component C) into antibody (100 µl)
- Add the antibody solution into Buccutite™ MTA vial (Component B)
- Incubate at room temperature for 30 minutes
- Mix with 50 µL Buccutite™ FOL-Activated PE-Cy5.5 (Component A)
- Incubate at room temperature for 60 minutes
PREPARATION OF WORKING SOLUTION
Antibody working solution
For labeling 100 µg antibody (assuming the target antibody concentration is 1 mg/mL), mix 5 µL (5% of the total reaction volume) of Reaction Buffer (Component C) with 100 µL of the target antibody solution.Note If you have a different concentration, adjust the antibody volume accordingly to make ~100 µg antibody available for your labeling reaction.
Note The antibody should be dissolved in 1X phosphate buffered saline (PBS), pH 7.2-7.4; If the antibody is dissolved in glycine buffer, it must be dialyzed against 1X PBS, pH 7.2-7.4, or use ReadiUse™ 10KD Spin Filter (Cat. # 60502 from AAT Bioquest) to remove free amines or ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for antibody precipitation.
Note Impure antibodies or antibodies stabilized with bovine serum albumin (BSA) or gelatin will not be labeled well.
Note The antibody –Buccutite™ MTA reaction efficiency is significantly reduced if the antibody concentration is less than 1 mg/mL. For optimal labeling efficiency the final antibody concentration range of 1-10 mg/mL is recommended.
SAMPLE EXPERIMENTAL PROTOCOL
Run Antibody-Buccutite™ MTA reaction
- Add the antibody working solution directly into the vial of Buccutite ™ MTA (Component B), and mix them well by repeatedly pipetting for a few times or vortex the vial for a few seconds.
- Keep the antibody- Buccutite ™ MTA reaction mixture at room temperature for 30 - 60 minutes.
Note The antibody-Buccutite™ MTA reaction mixture can be rotated or shaken for longer time if desired.
Make antibody-PE-Cy5.5 conjugation
- Make Buccutite™ FOL-Activated PE-Cy5.5 solution by adding 50 µL ddH2O into the vial of Buccutite™ FOL-Activated PE-Cy5.5 (Component A), mix well by repeatedly pipetting for a few times or vortex the vial for a few seconds.
- Mix whole vial of Buccutite™ FOL-Activated PE-Cy5.5 solution into the antibody-Buccutite™ MTA solution, mix well and rotating the mixture for 1 hour at room temperature.
- The antibody-PE-Cy5.5 conjugate is now ready to use.
Note For immediate use, the antibody-PE-Cy5.5 conjugate need be diluted with the buffer of your choice.
Storage of Antibody-PE-Cy5.5 Conjugate
The antibody conjugate should be stored at > 0.5 mg/mL in the presence of a carrier protein (e.g., 0.1% bovine serum albumin). The Antibody-PE-Cy5.5 conjugate solution could be stored at 4 °C for two months without significant change when stored in the presence of 2 mM sodium azide and kept from light. For longer storage, the antibody-PE-Cy5.5 conjugates could be lyophilized and stored at ≤ –20 °C.Table 1.Available fluorophores at AAT Bioquest Buccutite™ Rapid Antibody Labelling Kits
| Cat# | Labels | Ex (nm) | Em (nm) |
| 1310 | PE | 565 | 575 |
| 1322 | PE-Cy5 | 565 | 674 |
| 1316 | PE-Cy5.5 | 565 | 700 |
| 1317 | PE-Cy7 | 565 | 780 |
| 1318 | PE-Texas Red | 565 | 600 |
| 1311 | APC | 651 | 662 |
| 1319 | APC-iFluor™ 700 | 651 | 713 |
| 1320 | APC-Cy5.5 | 651 | 700 |
| 1321 | APC-Cy7 | 651 | 780 |
| 1325 | PerCP | 482 | 677 |
Spectrum
Open in Advanced Spectrum Viewer
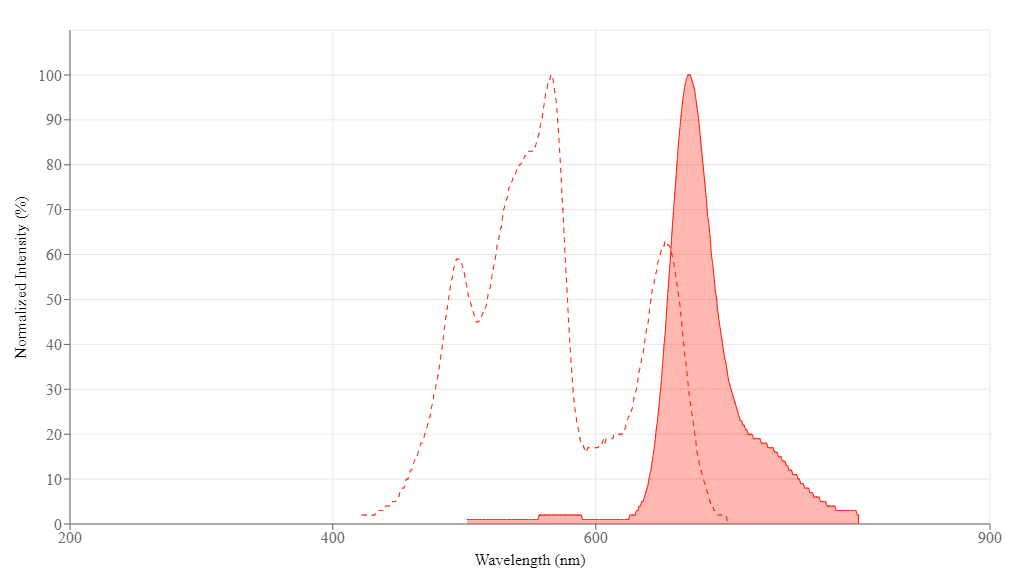

Spectral properties
| Extinction coefficient (cm -1 M -1) | 1960000 |
| Excitation (nm) | 565 |
| Emission (nm) | 671 |
Product Family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) |
| Buccutite™ Rapid APC-Cy5.5 Tandem Antibody Labeling Kit *Microscale Optimized for Labeling 100 ug Antibody Per Reaction* | 651 | 700 | 700000 |
| Buccutite™ Rapid APC-Cy5.5 Tandem Antibody Labeling Kit *Microscale Optimized for Labeling 25 ug Antibody Per Reaction* | 651 | 700 | 700000 |
| Buccutite™ Rapid APC-Cy5.5 Tandem Antibody Labeling Kit *Production Scale Optimized for Labeling 1 mg Antibody Per Reaction* | 651 | 700 | 700000 |
Images
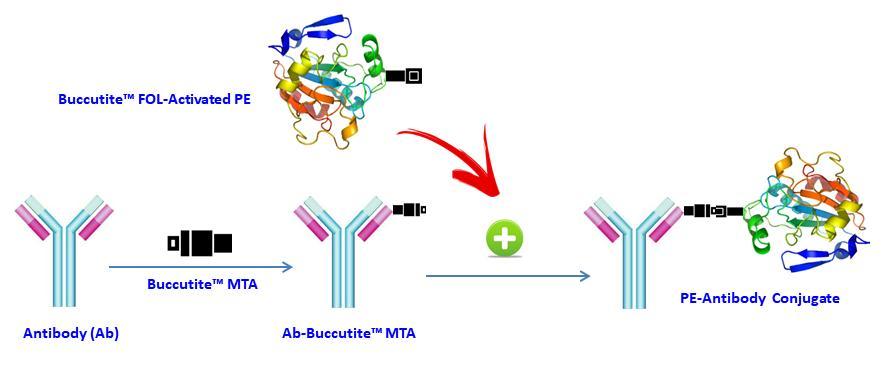
Figure 1. AAT Bioquest offers this Buccutite™ rapid labeling kit to facilitate the PE conjugations to antibodies and other proteins such as streptavidin and other secondary reagents. Our preactivated PE was premodified with our Buccutite™ FOL. Your antibody (or other proteins) is modified with our Buccutite™ MTA to give MTA-modified protein (such as antibody). The MTA-modified protein readily reacts with FOL-modified PE to give the desired PE-antibody conjugate in much higher yield than the SMCC chemistry. In addition, our preactivated PE reacts with MTA-modified biopolymers at much lower concentrations than the SMCC chemistry.
References
View all 46 references: Citation Explorer
Chromophore attachment to phycobiliprotein beta-subunits: phycocyanobilin:cysteine-beta84 phycobiliprotein lyase activity of CpeS-like protein from Anabaena Sp. PCC7120
Authors: Zhao KH, Su P, Li J, Tu JM, Zhou M, Bubenzer C, Scheer H.
Journal: J Biol Chem (2006): 8573
Authors: Zhao KH, Su P, Li J, Tu JM, Zhou M, Bubenzer C, Scheer H.
Journal: J Biol Chem (2006): 8573
Excitation energy transfer from phycobiliprotein to chlorophyll d in intact cells of Acaryochloris marina studied by time- and wavelength-resolved fluorescence spectroscopy
Authors: Petrasek Z, Schmitt FJ, Theiss C, Huyer J, Chen M, Larkum A, Eichler HJ, Kemnitz K, Eckert HJ.
Journal: Photochem Photobiol Sci (2005): 1016
Authors: Petrasek Z, Schmitt FJ, Theiss C, Huyer J, Chen M, Larkum A, Eichler HJ, Kemnitz K, Eckert HJ.
Journal: Photochem Photobiol Sci (2005): 1016
Single-molecule spectroscopy selectively probes donor and acceptor chromophores in the phycobiliprotein allophycocyanin
Authors: Loos D, Cotlet M, De Schryver F, Habuchi S, Hofkens J.
Journal: Biophys J (2004): 2598
Authors: Loos D, Cotlet M, De Schryver F, Habuchi S, Hofkens J.
Journal: Biophys J (2004): 2598
Isolation and characterisation of phycobiliprotein rich mutant of cyanobacterium Synechocystis sp
Authors: Prasanna R, Dhar DW, Dominic TK, Tiwari ON, Singh PK.
Journal: Acta Biol Hung (2003): 113
Authors: Prasanna R, Dhar DW, Dominic TK, Tiwari ON, Singh PK.
Journal: Acta Biol Hung (2003): 113
Evaluation of Tolypothrix germplasm for phycobiliprotein content
Authors: Prasanna R, Prasanna BM, Mohammadi SA, Singh PK.
Journal: Folia Microbiol (Praha) (2003): 59
Authors: Prasanna R, Prasanna BM, Mohammadi SA, Singh PK.
Journal: Folia Microbiol (Praha) (2003): 59
Co-ordinated expression of phycobiliprotein operons in the chromatically adapting cyanobacterium Calothrix PCC 7601: a role for RcaD and RcaG
Authors: Noubir S, Luque I, Ochoa de Alda JA, Perewoska I, T and eau de Marsac N, Cobley JG, Houmard J.
Journal: Mol Microbiol (2002): 749
Authors: Noubir S, Luque I, Ochoa de Alda JA, Perewoska I, T and eau de Marsac N, Cobley JG, Houmard J.
Journal: Mol Microbiol (2002): 749
Phycobiliprotein genes of the marine photosynthetic prokaryote Prochlorococcus: evidence for rapid evolution of genetic heterogeneity
Authors: Ting CS, Rocap G, King J, Chisholm SW.
Journal: Microbiology (2001): 3171
Authors: Ting CS, Rocap G, King J, Chisholm SW.
Journal: Microbiology (2001): 3171
Phycobiliprotein-Fab conjugates as probes for single particle fluorescence imaging
Authors: Triantafilou K, Triantafilou M, Wilson KM.
Journal: Cytometry (2000): 226
Authors: Triantafilou K, Triantafilou M, Wilson KM.
Journal: Cytometry (2000): 226
Novel activity of a phycobiliprotein lyase: both the attachment of phycocyanobilin and the isomerization to phycoviolobilin are catalyzed by the proteins PecE and PecF encoded by the phycoerythrocyanin operon
Authors: Zhao KH, Deng MG, Zheng M, Zhou M, Parbel A, Storf M, Meyer M, Strohmann B, Scheer H.
Journal: FEBS Lett (2000): 9
Authors: Zhao KH, Deng MG, Zheng M, Zhou M, Parbel A, Storf M, Meyer M, Strohmann B, Scheer H.
Journal: FEBS Lett (2000): 9
Phycobiliprotein and fluorescence immunological assay
Authors: Wu P., undefined
Journal: Sheng Li Ke Xue Jin Zhan (2000): 82
Authors: Wu P., undefined
Journal: Sheng Li Ke Xue Jin Zhan (2000): 82
Application notes
A New Protein Crosslinking Method for Labeling and Modifying Antibodies
A Novel Fluorescent Probe for Imaging and Detecting Hydroxyl Radical in Living Cells
A Novel NO Wash Probeniceid-Free Calcium Assay for Functional Analysis of GPCR and Calcium Channel Targets
Biotin Labeling Molecules and Their Biological Applications
Buccutite™ Bioconjugation Technology
A Novel Fluorescent Probe for Imaging and Detecting Hydroxyl Radical in Living Cells
A Novel NO Wash Probeniceid-Free Calcium Assay for Functional Analysis of GPCR and Calcium Channel Targets
Biotin Labeling Molecules and Their Biological Applications
Buccutite™ Bioconjugation Technology
FAQ
How can I lyse my cells without lysing the nuclear membrane?
What are the differences between calcium ion indicators: Cal 520, Cal 520FF, and Cal 520N?
How do I make an AM ester stock solution?
Can we fix cells with glutaraldehyde and then stain with fluorescent phalloidin?
What is the difference between FluoroQuest Anti-fading Kit I and FluoroQuest Anti-fading Kit II?
What are the differences between calcium ion indicators: Cal 520, Cal 520FF, and Cal 520N?
How do I make an AM ester stock solution?
Can we fix cells with glutaraldehyde and then stain with fluorescent phalloidin?
What is the difference between FluoroQuest Anti-fading Kit I and FluoroQuest Anti-fading Kit II?