C-PC [C-Phycocyanin] *CAS 11016-15-2*
Ordering information
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
Additional ordering information
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
Physical properties
| Molecular weight | ~264,000 |
| Solvent | Water |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Refrigerated (2-8 °C); Minimize light exposure |
| UNSPSC | 12171501 |
| Overview |
See also: PE and APC
CAS 11016-15-2 | Molecular weight ~264,000 |
C-Phycocyanin (C-PC) occurs as the major phycobiliprotein in many cyanobacteria and as a secondary phycobiliprotein in some red algae. The pigment has a single visible absorption maximum between 615 and 620 nm and a fluorescence emission maximum at ~650 nm. Its molecular weight is between 70,000 and 110,000 daltons. The pigment is composed of two subunits, ? and ?, which occur in equal numbers, but the exact number of ? and ? pairs which make up the molecule may vary among the species. Both ? and ? subunits contain only the PCB chromophore. In addition to absorbing light directly, this intensely blue pigment accepts quanta from phycoerythrin by fluorescence energy transfer in organisms in which PE is present. The red fluorescence of C-PC is transferred to allophycocyanin.
Images
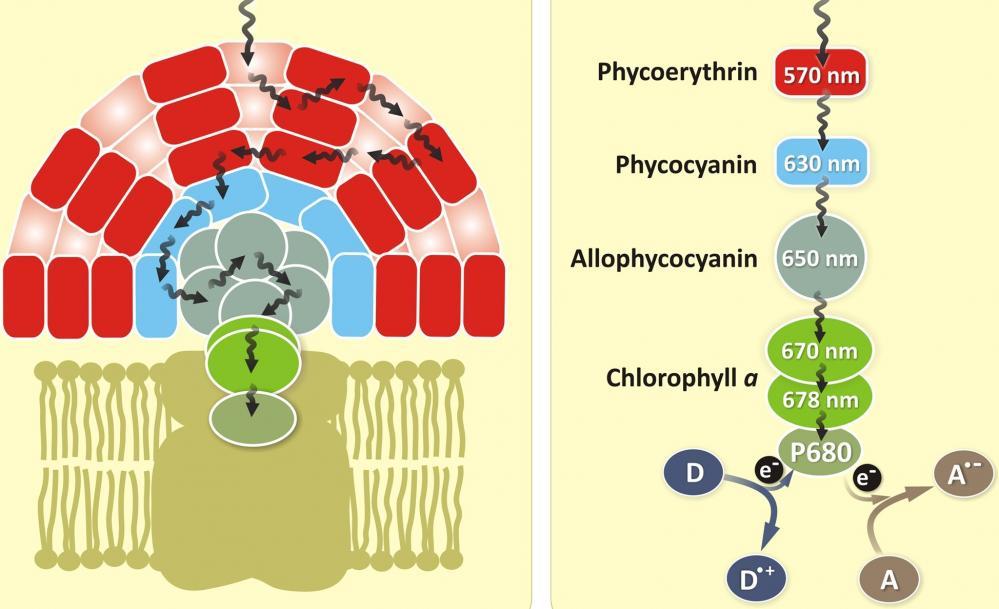
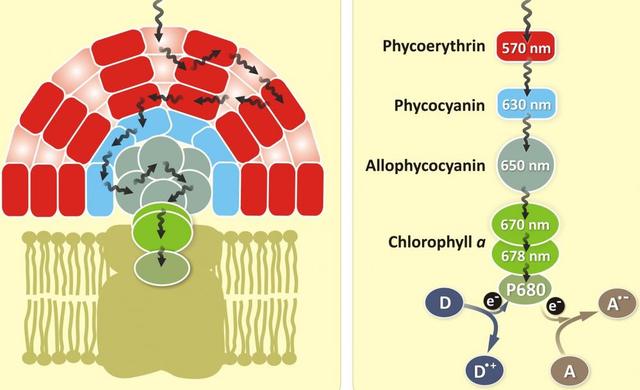
Figure 1. Phycocyanin is a protein from the light-harvesting phycobiliprotein family, along with allophycocyanin, phycoerythrin and phycoerythrocyanin. It is an accessory pigment to chlorophyll. All phycobiliproteins are water-soluble and therefore cannot exist within the membrane like carotenoids, but aggregate forming clusters that adhere to the membrane called phycobilisomes. Allophycocyanin absorbs and emits red light, and is readily found in Cyanobacteria, and red algae. Phycobilin pigments have excellent fluorescent properties that are extremely useful for flow cytometry-based immunoassays.
Citations
View all 2 citations: Citation Explorer
Small-scale Production and Business Plan for Phycocyanin from Cyanobacteria
Authors: Nazar, Reehana and Yousuff, Mohamed Imran Mohamed and Nooruddin, Thajuddin and Dharumadurai, Dhanasekaran
Journal: (2023): 253--277
Authors: Nazar, Reehana and Yousuff, Mohamed Imran Mohamed and Nooruddin, Thajuddin and Dharumadurai, Dhanasekaran
Journal: (2023): 253--277
An automated, modular system for organic waste utilization using heterotrophic alga Galdieria sulphuraria: Design considerations and sustainability
Authors: Pahmeyer, Maximilian Julius and Siddiqui, Shahida Anusha and Pleissner, Daniel and Go{\l}aszewski, Janusz and Heinz, Volker and Smetana, Sergiy
Journal: Bioresource Technology (2022): 126800
Authors: Pahmeyer, Maximilian Julius and Siddiqui, Shahida Anusha and Pleissner, Daniel and Go{\l}aszewski, Janusz and Heinz, Volker and Smetana, Sergiy
Journal: Bioresource Technology (2022): 126800
References
View all 46 references: Citation Explorer
Chromophore attachment to phycobiliprotein beta-subunits: phycocyanobilin:cysteine-beta84 phycobiliprotein lyase activity of CpeS-like protein from Anabaena Sp. PCC7120
Authors: Zhao KH, Su P, Li J, Tu JM, Zhou M, Bubenzer C, Scheer H.
Journal: J Biol Chem (2006): 8573
Authors: Zhao KH, Su P, Li J, Tu JM, Zhou M, Bubenzer C, Scheer H.
Journal: J Biol Chem (2006): 8573
Excitation energy transfer from phycobiliprotein to chlorophyll d in intact cells of Acaryochloris marina studied by time- and wavelength-resolved fluorescence spectroscopy
Authors: Petrasek Z, Schmitt FJ, Theiss C, Huyer J, Chen M, Larkum A, Eichler HJ, Kemnitz K, Eckert HJ.
Journal: Photochem Photobiol Sci (2005): 1016
Authors: Petrasek Z, Schmitt FJ, Theiss C, Huyer J, Chen M, Larkum A, Eichler HJ, Kemnitz K, Eckert HJ.
Journal: Photochem Photobiol Sci (2005): 1016
Single-molecule spectroscopy selectively probes donor and acceptor chromophores in the phycobiliprotein allophycocyanin
Authors: Loos D, Cotlet M, De Schryver F, Habuchi S, Hofkens J.
Journal: Biophys J (2004): 2598
Authors: Loos D, Cotlet M, De Schryver F, Habuchi S, Hofkens J.
Journal: Biophys J (2004): 2598
Isolation and characterisation of phycobiliprotein rich mutant of cyanobacterium Synechocystis sp
Authors: Prasanna R, Dhar DW, Dominic TK, Tiwari ON, Singh PK.
Journal: Acta Biol Hung (2003): 113
Authors: Prasanna R, Dhar DW, Dominic TK, Tiwari ON, Singh PK.
Journal: Acta Biol Hung (2003): 113
Evaluation of Tolypothrix germplasm for phycobiliprotein content
Authors: Prasanna R, Prasanna BM, Mohammadi SA, Singh PK.
Journal: Folia Microbiol (Praha) (2003): 59
Authors: Prasanna R, Prasanna BM, Mohammadi SA, Singh PK.
Journal: Folia Microbiol (Praha) (2003): 59
Co-ordinated expression of phycobiliprotein operons in the chromatically adapting cyanobacterium Calothrix PCC 7601: a role for RcaD and RcaG
Authors: Noubir S, Luque I, Ochoa de Alda JA, Perewoska I, T and eau de Marsac N, Cobley JG, Houmard J.
Journal: Mol Microbiol (2002): 749
Authors: Noubir S, Luque I, Ochoa de Alda JA, Perewoska I, T and eau de Marsac N, Cobley JG, Houmard J.
Journal: Mol Microbiol (2002): 749
Phycobiliprotein genes of the marine photosynthetic prokaryote Prochlorococcus: evidence for rapid evolution of genetic heterogeneity
Authors: Ting CS, Rocap G, King J, Chisholm SW.
Journal: Microbiology (2001): 3171
Authors: Ting CS, Rocap G, King J, Chisholm SW.
Journal: Microbiology (2001): 3171
Phycobiliprotein-Fab conjugates as probes for single particle fluorescence imaging
Authors: Triantafilou K, Triantafilou M, Wilson KM.
Journal: Cytometry (2000): 226
Authors: Triantafilou K, Triantafilou M, Wilson KM.
Journal: Cytometry (2000): 226
Novel activity of a phycobiliprotein lyase: both the attachment of phycocyanobilin and the isomerization to phycoviolobilin are catalyzed by the proteins PecE and PecF encoded by the phycoerythrocyanin operon
Authors: Zhao KH, Deng MG, Zheng M, Zhou M, Parbel A, Storf M, Meyer M, Strohmann B, Scheer H.
Journal: FEBS Lett (2000): 9
Authors: Zhao KH, Deng MG, Zheng M, Zhou M, Parbel A, Storf M, Meyer M, Strohmann B, Scheer H.
Journal: FEBS Lett (2000): 9
Phycobiliprotein and fluorescence immunological assay
Authors: Wu P., undefined
Journal: Sheng Li Ke Xue Jin Zhan (2000): 82
Authors: Wu P., undefined
Journal: Sheng Li Ke Xue Jin Zhan (2000): 82
Application notes
Phycobiliproteins and Their Fluorescent Labeling Applications
A New Protein Crosslinking Method for Labeling and Modifying Antibodies
A Novel Fluorescent Probe for Imaging and Detecting Hydroxyl Radical in Living Cells
Buccutite™ Bioconjugation Technology
Monitoring of Mitochondrial Membrane Potential Changes in Live Cells Using JC-10
A New Protein Crosslinking Method for Labeling and Modifying Antibodies
A Novel Fluorescent Probe for Imaging and Detecting Hydroxyl Radical in Living Cells
Buccutite™ Bioconjugation Technology
Monitoring of Mitochondrial Membrane Potential Changes in Live Cells Using JC-10
FAQ
Do you have any dual-fluorescence nucleic acid stains that interact with both DNA and RNA?
How do I stain T cells with CytoTell™ Blue?
What is the number of passages your Cell Explorer Live Cell Tracking assays can undergo?
Can I intracellularly measure mitochondria calcium flux and changes in mitochondria membrane potential at the same time?
Do you have fixable live cell staining assay kits?
How do I stain T cells with CytoTell™ Blue?
What is the number of passages your Cell Explorer Live Cell Tracking assays can undergo?
Can I intracellularly measure mitochondria calcium flux and changes in mitochondria membrane potential at the same time?
Do you have fixable live cell staining assay kits?