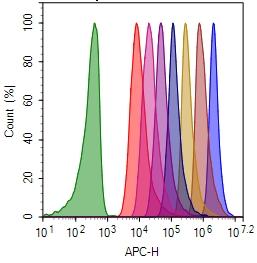
CytoTell™ Red 650
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
| Molecular weight | ~600 |
| Solvent | DMSO |
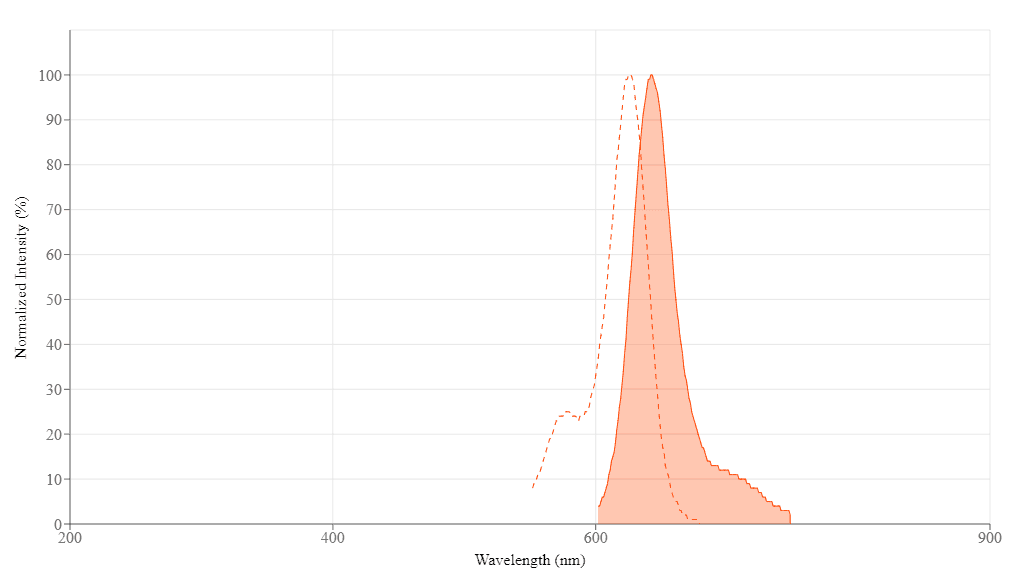
| Excitation (nm) | 626 |
| Emission (nm) | 643 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
| Overview |
Molecular weight ~600 | Excitation (nm) 626 | Emission (nm) 643 |
Platform
Flow cytometer
| Excitation | 640 nm laser |
| Emission | 660/20 nm filter |
| Instrument specification(s) | APC channel |
Example protocol
AT A GLANCE
Protocol summary
- Prepare cells with test compounds
- Add 1X dye working solution
- Incubate dyes with cells at room temperature or 37 oC for 10 to 30 minutes
- Remove the dye working solution
- Analyse with flow cytometer with appropriate filter set
Important notes
Bring all the kit components at room temperature before starting the experiment. Note: The CytoTell™ dyes are lyophilized powders. They should be stable for at least 6 months if store at -20 °C, protecting from light, and avoiding freeze/thaw cycles.
|
Product Number |
Indicator |
Size |
Ex/Em (nm) |
Excitation Source |
|
22240 |
CytoTell™ UltraGreen |
500 tests |
492/519 |
488 nm (Blue Laser) |
|
22241 |
CytoTell™ UltraGreen |
1000 tests |
492/519 |
488 nm (Blue Laser) |
|
22248 |
CytoTell™ Violet 500 |
500 tests |
415/499 |
405 nm (Violet Laser) |
|
22251 |
CytoTell™ Blue |
500 tests |
403/454 |
405 nm (Violet Laser) |
|
22252 |
CytoTell™ Blue |
1000 tests |
403/454 |
405 nm (Violet Laser) |
|
22253 |
CytoTell™ Green |
500 tests |
511/525 |
488 nm (Blue Laser) |
|
22254 |
CytoTell™ Green |
1000 tests |
511/525 |
488 nm (Blue Laser) |
|
22255 |
CytoTell™ Red 650 |
500 tests |
628/643 |
633 nm (Red Laser) |
|
22256 |
CytoTell™ Red 650 |
1000 tests |
628/643 |
633 nm (Red Laser) |
|
22257 |
CytoTell™ Orange |
500 tests |
542 /556 |
488 nm (Blue Laser) |
|
22258 |
CytoTell™ Orange |
1000 tests |
542 /556 |
488 nm (Blue Laser) |
|
22261 |
CytoTell™ Red 590 |
500 tests |
560 /574 |
488 nm (Blue Laser) |
|
22262 |
CytoTell™ Red 590 |
1000 tests |
560 /574 |
488 nm (Blue Laser) |
PREPARATION OF STOCK SOLUTION
CytoTell™ dye stock solution (500X):
Add 500 µL DMSO into the dye powder vial, mix it well by vortexing to have a stock solution (500X). Note: The stock solution should be used promptly; any remaining solution should be aliquoted and frozen at < - 20 oC. Avoid repeated freeze-thaw cycles, and protect from light.
PREPARATION OF WORKING SOLUTION
CytoTellTM dye working solution (1X):
Dilute the 500X DMSO stock solution at 1 to 500 in Hanks and 20 mM Hepes buffer (HHBS) or the buffer of your choice, pH 7 (such as 1 µL of 500X DMSO stock solution to 500 µL buffer) right before use. Mix them well by vortexing. Note: The final concentration of the dye working solution should be empirically determined for different cell types and/or experimental conditions. It is recommended to test at the concentrations that are at least over ten fold range. Such as CytoTell™ Red might use much less amount in some cell types than the recommend concentrations.
SAMPLE EXPERIMENTAL PROTOCOL
- Treat cells with test compounds for a desired period of time.
- Centrifuge the cells to get 1-5 × 105 cells per tube.
- Resuspend cells in 500 µL of the CytoTell™ dye working solution. Optional: One can add the 500X DMSO stock solution into the cells directly without medium removing (such as, add 1 µL500X DMSO stock solution into 500 µL cells)
- Incubate cells with a dye solution at room temperature or 37 °C for 10 to 30 minutes, protected from light.
- Remove the dye working solution from the cells, wash the cells with HHBS or buffer of your choice. Resuspend cells in 500 µL of pre-warmed HHBS or medium to get 1-5 × 105 cells per tube.
- Monitor the fluorescence change at respected Ex/Em (see Table 1) with a flow cytometer or a fluorescence microscope.
Images
Citations
Authors: Katsuyama, Naoya and Kawase, Takakazu and Barakat, Carolyne and Mizuno, M and Tomita, Akihiro and Ozeki, Kazutaka and Nishio, Nobuhiro and Sato, Yoshie and Kajiya, Ryoko and Shiraishi, Keiko and others,
Journal: Nagoya J Med Sci (2023)
Authors: Barakat, Carolyne and Inagaki, Yuichiro and Mizuno, Shohei and Nishio, Nobuhiro and Katsuyama, Naoya and Sato, Yoshie and Kobayashi, Miki and Ozeki, Kazutaka and Iida, Hiroatsu and Tomita, Akihiro and others,
Journal: International Journal of Hematology (2023): 1--15
Authors: Ishihara, Mikiya and Miwa, Hiroshi and Fujiwara, Hiroshi and Akahori, Yasushi and Kato, Takuma and Tanaka, Yoshimasa and Tawara, Isao and Shiku, Hiroshi
Journal: iScience (2023)
Authors: Huang, Cheng and Li, Hongjian and Feng, Yunyu and Li, Xiaoling and Zhang, Zongliang and Jiang, Caiying and Wang, Jichao and Yang, Chenli and Fu, Yuying and Mu, Min and others,
Journal: Theranostics (2020): 10498
Authors: Ozay, E Ilker and Shanthalingam, Sudarvili and Sherman, Heather L and Torres, Joe A and Osborne, Barbara A and Tew, Gregory N and Minter, Lisa M
Journal: Molecular Therapy (2020): 1987--2006
Authors: Palacio-Casta{\~n}eda, Valentina and Kooijman, Lucas and Venzac, Bastien and Verdurmen, Wouter PR and Le Gac, S{\'e}verine
Journal: Micromachines (2020): 382
Authors: Govindan, Subashika and Oberst, Polina and Jabaudon, Denis
Journal: Nature protocols (2018): 2297--2311
Authors: Klöss, Volker
Journal: (2017)
Authors: Klöss, Volker and Grünvogel, Oliver and Wabnitz, Guido and Eigenbrod, Tatjana and Ehrhardt, Stefanie and Lasitschka, Felix and Lohmann, Volker and Dalpke, Alex and er H, undefined
Journal: Frontiers in Immunology (2017): 1238
Authors: Minami, Hirohito and Nagaharu, Keiki and Nakamori, Yoshiki and Ohishi, Kohshi and Shimojo, Naoshi and Kageyama, Yuki and Matsumoto, Takeshi and Sugimoto, Yuka and Tawara, Isao and Masuya, Masahiro and others, undefined
Journal: The Journal of Immunology (2017): ji1700054
Application notes
Relative Brightness of Fluorescent Dyes
A New Protein Crosslinking Method for Labeling and Modifying Antibodies
A Novel Fluorescent Probe for Imaging and Detecting Hydroxyl Radical in Living Cells
Buccutite™ Bioconjugation Technology
FAQ
AssayWise
Unlocking the Potential of 3D Cell Culture: A Guide to Assay Optimization
Buccutite™ Conjugation Kits: Quick and Easy Antibody Labeling
Nucleic Acid Detection, Quantification and Imaging
A practical guide for use of PE and APC in flow cytometry