D-Luciferin, potassium salt *CAS#: 115144-35-9*
Ordering information
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
Additional ordering information
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
Physical properties
| Molecular weight | 318.41 |
| Solvent | Water |
Spectral properties
| Excitation (nm) | 385 |
| Emission (nm) | 529 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 41105331 |
| Overview |
See also: Luciferases, Oxidases
CAS 115144-35-9 | Molecular weight 318.41 | Excitation (nm) 385 | Emission (nm) 529 |
Luciferin is the most popular and versatile bioluminescent substrate. The firefly luciferase/luciferin bioluminescent system is found in the firefly (Photinus pyralis) and several other beetles. Luciferase oxidizes ATP-activated luciferin through a dioxetanone intermediate. Firefly luciferase produces light by the ATP-dependent oxidation of luciferin. The 560 nm chemiluminescence from this reaction peaks within seconds, with light output that is proportional to luciferase activity when luciferin and ATP are present in excess. Firefly luciferase has long been conjugated to antibodies and used as a label in immunoassays using luciferin as the substrate for detection. Compared to HRP and alkaline phosphatase, luciferase is less tolerant to chemical modifications. One particular advantage to the enzyme is that there is low endogenous luciferase activity in mammalian tissues besides its high sensitivity. Another important use of luciferase is in the area of hygiene monitoring. The luciferase/luciferin system can be used to detect contamination because ATP, present in all living organisms, is required to produce luminescence. The main application for this type of ATP bioluminescence is quality assurance by testing surfacesin food processing plants to determine whether or not there iscontamination of eitherequipment or products.
Calculators
Common stock solution preparation
Table 1. Volume of Water needed to reconstitute specific mass of D-Luciferin, potassium salt *CAS#: 115144-35-9* to given concentration. Note that volume is only for preparing stock solution. Refer to sample experimental protocol for appropriate experimental/physiological buffers.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 314.06 µL | 1.57 mL | 3.141 mL | 15.703 mL | 31.406 mL |
| 5 mM | 62.812 µL | 314.06 µL | 628.121 µL | 3.141 mL | 6.281 mL |
| 10 mM | 31.406 µL | 157.03 µL | 314.06 µL | 1.57 mL | 3.141 mL |
Molarity calculator
Enter any two values (mass, volume, concentration) to calculate the third.
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Images
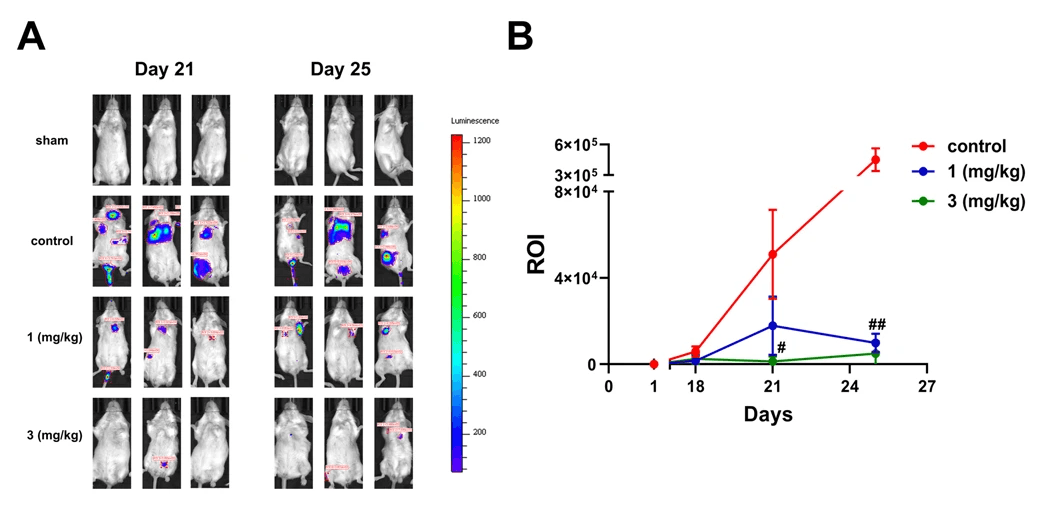
Figure 2. Diltiazem attenuates 4T1 cells colonization to lung in vivo. A Representative IVIS images of BALB/c mice 21 days after 4T1-luc cells injection. Diltiazem (1, 3 mg/kg) was given by oral gavage, and ddH2O was served as vehicle control. B Quantification of luciferase intensity of mice on days 18, 21, and 25. Graphs showed mean ± S.D. of at least four independent experiments. p value was calculated using Student’s t test. ***p < 0.001 compared to sham group. ###p < 0.001 compared to control group. Source: Diltiazem inhibits breast cancer metastasis via mediating growth differentiation factor 15 and epithelial-mesenchymal transition by Yen-Chang Chen, Chen-Teng Wu, Jia-Hong Chen, Cheng-Fang Tsai, Chen-Yun Wu, Pei-Chun Chang & Wei-Lan Yeh. Oncogenesis. August 2022.
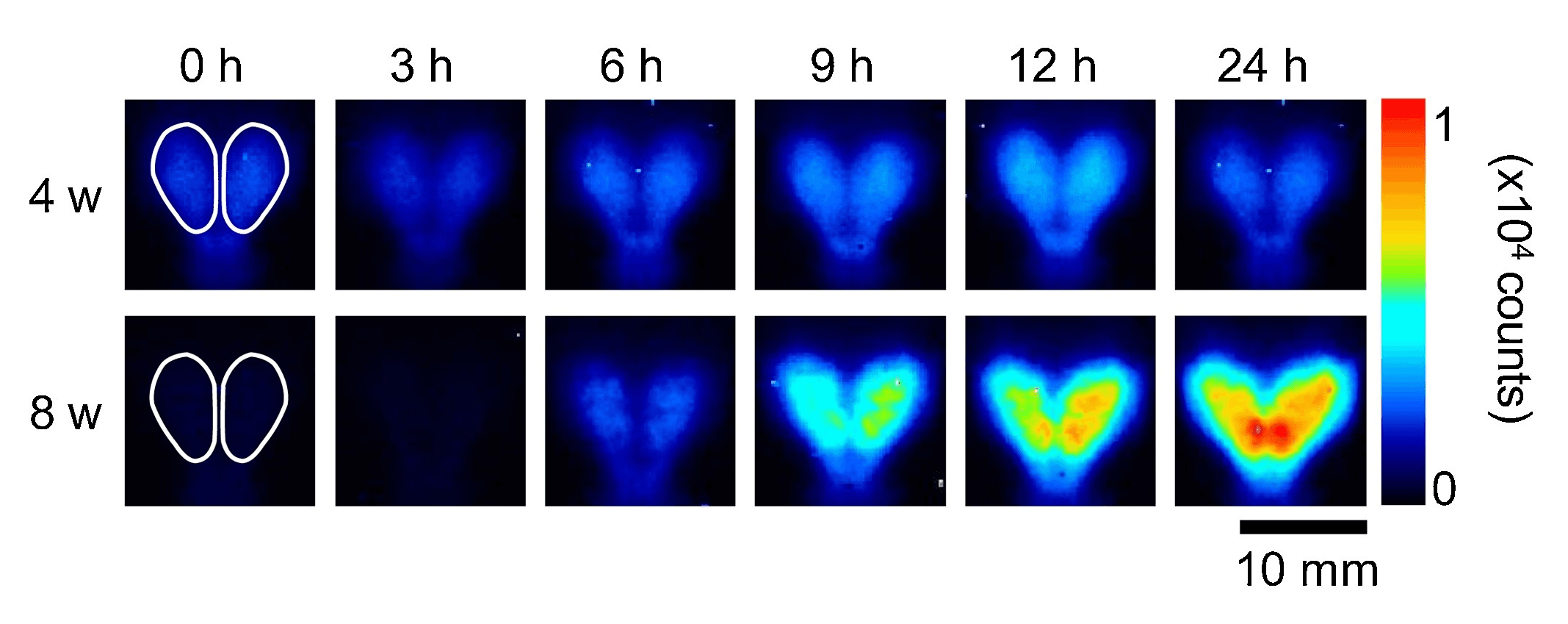
Figure 3. Representative images of photon emission in Arc-Luc Tg HL mice at 4 W and 8 W with acute GLA treatment (80 mg/kg BW i.p.). Mice were subcutaneously implanted with luciferin-filled osmotic pumps and bioluminescence signal images in the forebrain (pseudocolored, 0–10,000 counts) were examined before (at 0 h) and after (at 3, 6, 9, 12, and 24 h) acute GLA treatment. Location of ROIs for the forebrain (white circles) is indicated in images at 0 h. Scale bar, 10 mm. Source: Bioluminescence imaging of Arc expression in mouse brain under acute and chronic exposure to pesticides by Hironori Izumi, Tetsuya Ishimoto, Hiroshi Yamamoto, and Hisashi Mori. NeuroToxicology. March 2019.
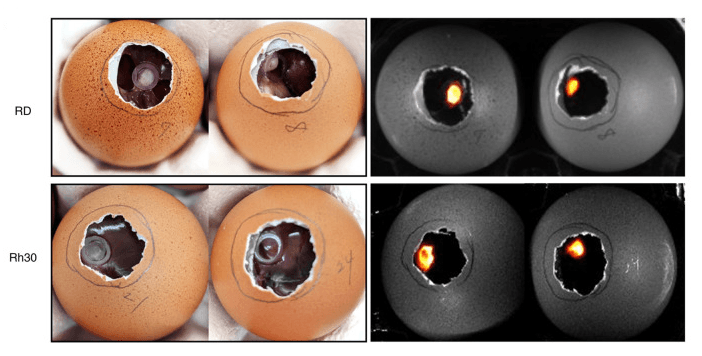
Figure 4. Establishment of a cell-derived xenograft model on the CAM using the RMS cell lines, RD and Rh30. A total of 7 days after transplantation with firefly-expressing RD and Rh30 cells onto the CAM, both tumors formed a mass that could be visualized by adding D-luciferin. Tumor formed on the CAM on day 16 (7 days after transplantation of RD or Rh30 cells). Images on the left were captured on a clean bench, whereas images on the right were observed using the G:BOX Chemi XRQ gel doc system following the addition of luciferin. Source: In ovo chorioallantoic membrane assay as a xenograft model for pediatric rhabdomyosarcoma by Chika Shoji, Ken Kikuchi, Hideki Yoshida, Mitsuru Miyachi, Shigeki Yagyu, Kunihiko Tsuchiya, Takaaki Nakaya, Hajime Hosoi, and Tomoko Iehara. Oncology Reports, March 2023.
Citations
View all 19 citations: Citation Explorer
Tonic TCR and IL-1$\beta$ signaling mediate phenotypic alterations of naive CD4+ T cells
Authors: Sekiya, Takashi and Hidano, Shinya and Takaki, Satoshi
Journal: Cell Reports (2024): 113954
Authors: Sekiya, Takashi and Hidano, Shinya and Takaki, Satoshi
Journal: Cell Reports (2024): 113954
Water Quality Monitoring with the Multiplexed Assay MitoOxTox for Mitochondrial Toxicity, Oxidative Stress Response, and Cytotoxicity in AREc32 Cells
Authors: Lee, Jungeun and König, Maria and Braun, Georg and Escher, Beate I
Journal: Environmental Science \& Technology (2024)
Authors: Lee, Jungeun and König, Maria and Braun, Georg and Escher, Beate I
Journal: Environmental Science \& Technology (2024)
GRAS family member LATERAL SUPPRESSOR regulates the initiation and morphogenesis of watermelon lateral organs
Authors: Jiang, Yanxin and Zhang, Anran and He, Wenjing and Li, Qingqing and Zhao, Bosi and Zhao, Hongjiao and Ke, Xubo and Guo, Yalu and Sun, Piaoyun and Yang, Tongwen and others,
Journal: Plant Physiology (2023): kiad445
Authors: Jiang, Yanxin and Zhang, Anran and He, Wenjing and Li, Qingqing and Zhao, Bosi and Zhao, Hongjiao and Ke, Xubo and Guo, Yalu and Sun, Piaoyun and Yang, Tongwen and others,
Journal: Plant Physiology (2023): kiad445
A rat model of dual-flow liver machine perfusion system
Authors: Ohara, Masayuki and Ishikawa, Jun and Yoshimoto, Syuhei and Hakamata, Yoji and Kobayashi, Eiji
Journal: Acta Cir{\'u}rgica Brasileira (2023): e387723
Authors: Ohara, Masayuki and Ishikawa, Jun and Yoshimoto, Syuhei and Hakamata, Yoji and Kobayashi, Eiji
Journal: Acta Cir{\'u}rgica Brasileira (2023): e387723
In ovo chorioallantoic membrane assay as a xenograft model for pediatric rhabdomyosarcoma
Authors: Shoji, Chika and Kikuchi, Ken and Yoshida, Hideki and Miyachi, Mitsuru and Yagyu, Shigeki and Tsuchiya, Kunihiko and Nakaya, Takaaki and Hosoi, Hajime and Iehara, Tomoko
Journal: Oncology Reports (2023): 1--10
Authors: Shoji, Chika and Kikuchi, Ken and Yoshida, Hideki and Miyachi, Mitsuru and Yagyu, Shigeki and Tsuchiya, Kunihiko and Nakaya, Takaaki and Hosoi, Hajime and Iehara, Tomoko
Journal: Oncology Reports (2023): 1--10
Andrographolide Derivatives Target the KEAP1/NRF2 Axis and Possess Potent Anti-SARS-CoV-2 Activity
Authors: Schulte, Bianca and K{\"o}nig, Maria and Escher, Beate I and Wittenburg, Sophie and Proj, Matic and Wolf, Valentina and Lemke, Carina and Schnakenburg, Gregor and Sosi{\v{c}}, Izidor and Streeck, Hendrik and others,
Journal: ChemMedChem (2022): e202100732
Authors: Schulte, Bianca and K{\"o}nig, Maria and Escher, Beate I and Wittenburg, Sophie and Proj, Matic and Wolf, Valentina and Lemke, Carina and Schnakenburg, Gregor and Sosi{\v{c}}, Izidor and Streeck, Hendrik and others,
Journal: ChemMedChem (2022): e202100732
Magnetically empowered bone marrow cells as a micro-living motor can improve early hematopoietic reconstitution
Authors: Mai, Qiusui and Wang, Zhengyuan and Chen, Quanfeng and Zhang, Jialu and Zhang, Dingyi and Li, Chengyao and Jiang, Qianli
Journal: Cytotherapy (2022)
Authors: Mai, Qiusui and Wang, Zhengyuan and Chen, Quanfeng and Zhang, Jialu and Zhang, Dingyi and Li, Chengyao and Jiang, Qianli
Journal: Cytotherapy (2022)
Diltiazem inhibits breast cancer metastasis via mediating growth differentiation factor 15 and epithelial-mesenchymal transition
Authors: Chen, Yen-Chang and Wu, Chen-Teng and Chen, Jia-Hong and Tsai, Cheng-Fang and Wu, Chen-Yun and Chang, Pei-Chun and Yeh, Wei-Lan
Journal: Oncogenesis (2022): 1--9
Authors: Chen, Yen-Chang and Wu, Chen-Teng and Chen, Jia-Hong and Tsai, Cheng-Fang and Wu, Chen-Yun and Chang, Pei-Chun and Yeh, Wei-Lan
Journal: Oncogenesis (2022): 1--9
Cytotoxicity Burst? Differentiating Specific from Nonspecific Effects in Tox21 in Vitro Reporter Gene Assays
Authors: Escher, Beate I and Henneberger, Luise and K{\"o}nig, Maria and Schlichting, Rita and Fischer, Fabian C
Journal: Environmental health perspectives (2020): 077007
Authors: Escher, Beate I and Henneberger, Luise and K{\"o}nig, Maria and Schlichting, Rita and Fischer, Fabian C
Journal: Environmental health perspectives (2020): 077007
IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression
Authors: Sadik, Ahmed and Patterson, Luis F Somarribas and {\"O}zt{\"u}rk, Selcen and Mohapatra, Soumya R and Panitz, Verena and Secker, Philipp F and Pf{\"a}nder, Pauline and Loth, Stefanie and Salem, Heba and Prentzell, Mirja Tamara and others,
Journal: Cell (2020): 1252--1270
Authors: Sadik, Ahmed and Patterson, Luis F Somarribas and {\"O}zt{\"u}rk, Selcen and Mohapatra, Soumya R and Panitz, Verena and Secker, Philipp F and Pf{\"a}nder, Pauline and Loth, Stefanie and Salem, Heba and Prentzell, Mirja Tamara and others,
Journal: Cell (2020): 1252--1270
References
View all 113 references: Citation Explorer
Mutagenesis of solvent-exposed amino acids in Photinus pyralis luciferase improves thermostability and pH-tolerance
Authors: Law GH, G and elman OA, Tisi LC, Lowe CR, Murray JA.
Journal: Biochem J (2006): 305
Authors: Law GH, G and elman OA, Tisi LC, Lowe CR, Murray JA.
Journal: Biochem J (2006): 305
Determination of p-glycoprotein ATPase activity using luciferase
Authors: Matsunaga T, Kose E, Yasuda S, Ise H, Ikeda U, Ohmori S.
Journal: Biol Pharm Bull (2006): 560
Authors: Matsunaga T, Kose E, Yasuda S, Ise H, Ikeda U, Ohmori S.
Journal: Biol Pharm Bull (2006): 560
Expression of firefly luciferase in Candida albicans and its use in the selection of stable transformants
Authors: Doyle TC, Nawotka KA, Purchio AF, Akin AR, Francis KP, Contag PR.
Journal: Microb Pathog (2006): 69
Authors: Doyle TC, Nawotka KA, Purchio AF, Akin AR, Francis KP, Contag PR.
Journal: Microb Pathog (2006): 69
PET imaging and optical imaging with D-luciferin [11C]methyl ester and D-luciferin [11C]methyl ether of luciferase gene expression in tumor xenografts of living mice
Authors: Wang JQ, Pollok KE, Cai S, Stantz KM, Hutchins GD, Zheng QH.
Journal: Bioorg Med Chem Lett (2006): 331
Authors: Wang JQ, Pollok KE, Cai S, Stantz KM, Hutchins GD, Zheng QH.
Journal: Bioorg Med Chem Lett (2006): 331
Bioluminescence of monolayers of firefly luciferase immobilized on graphite
Authors: Palomba S, Berovic N, Palmer RE.
Journal: Langmuir (2006): 5451
Authors: Palomba S, Berovic N, Palmer RE.
Journal: Langmuir (2006): 5451
Bovine serum albumin displays luciferase-like activity in presence of luciferyl adenylate: insights on the origin of protoluciferase activity and bioluminescence colours
Authors: Viviani VR, Ohmiya Y.
Journal: Luminescence (2006): 262
Authors: Viviani VR, Ohmiya Y.
Journal: Luminescence (2006): 262
Noninvasive bioluminescence imaging of luciferase expressing intracranial U87 xenografts: correlation with magnetic resonance imaging determined tumor volume and longitudinal use in assessing tumor growth and antiangiogenic treatment effect
Authors: Szentirmai O, Baker CH, Lin N, Szucs S, Takahashi M, Kiryu S, Kung AL, Mulligan RC, Carter BS.
Journal: Neurosurgery (2006): 365
Authors: Szentirmai O, Baker CH, Lin N, Szucs S, Takahashi M, Kiryu S, Kung AL, Mulligan RC, Carter BS.
Journal: Neurosurgery (2006): 365
Two different domains of the luciferase gene in the heterotrophic dinoflagellate Noctiluca scintillans occur as two separate genes in photosynthetic species
Authors: Liu L, Hastings JW.
Journal: Proc Natl Acad Sci U S A. (2006)
Authors: Liu L, Hastings JW.
Journal: Proc Natl Acad Sci U S A. (2006)
Effect of different salts and detergents on luciferin-luciferase luminescence of the enchytraeid Fridericia heliota
Authors: Rodionova NS, Petushkov VN.
Journal: J Photochem Photobiol B (2006): 123
Authors: Rodionova NS, Petushkov VN.
Journal: J Photochem Photobiol B (2006): 123
Evaluation of firefly luciferase bioluminescence mediated photodynamic toxicity in cancer cells
Authors: Schipper ML, Patel MR, Gambhir SS.
Journal: Mol Imaging Biol (2006): 218
Authors: Schipper ML, Patel MR, Gambhir SS.
Journal: Mol Imaging Biol (2006): 218
Application notes
A New Protein Crosslinking Method for Labeling and Modifying Antibodies
Selective Detection of Pyrophosphate Using a Fluorogenic Pyrophosphate Sensor
Restriction of Advanced Glycation End Products Improves Insulin Resistance in Human Type 2 Diabetes
Matrix Remodeling Maintains Embryonic Stem Cell Self-Renewal by Activating Stat3
Human High Temperature Requirement Serine Protease A1 (HTRA1) Degrades Tau Protein Aggregates
Selective Detection of Pyrophosphate Using a Fluorogenic Pyrophosphate Sensor
Restriction of Advanced Glycation End Products Improves Insulin Resistance in Human Type 2 Diabetes
Matrix Remodeling Maintains Embryonic Stem Cell Self-Renewal by Activating Stat3
Human High Temperature Requirement Serine Protease A1 (HTRA1) Degrades Tau Protein Aggregates
FAQ
What is the best way to administer D-Luciferin potassium salt for in vivo bioluminescent imaging?
Why should I use an absorbance ratio at A575nm/A605nm when using most of your Amplite® Colorimetric Assay Kits?
How should I reconstitute an NADPH standard?
Will Amplite® Fluorimetric NAD/NADH Ratio Assay Kit *Red Fluorescence* work with NADP/NADPH? Can this kit measure NADP+ and NADPH?
What assay kits measure NADP/NADPH from cell samples?
Why should I use an absorbance ratio at A575nm/A605nm when using most of your Amplite® Colorimetric Assay Kits?
How should I reconstitute an NADPH standard?
Will Amplite® Fluorimetric NAD/NADH Ratio Assay Kit *Red Fluorescence* work with NADP/NADPH? Can this kit measure NADP+ and NADPH?
What assay kits measure NADP/NADPH from cell samples?