Amplite® Colorimetric BCA Protein Quantitation Assay Kit
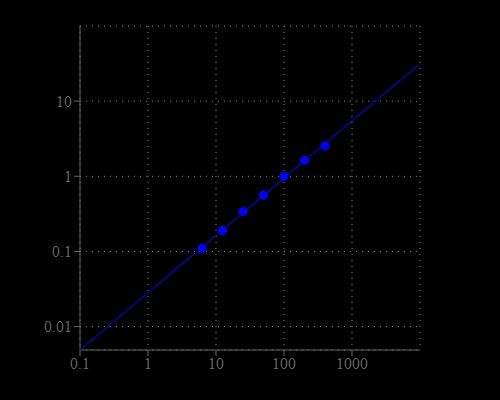
Amplite® Colorimetric BCA Protein Quantitation Assay Kit delivers rapid and accurate protein quantitation without the need for heating or long incubation.
- Rapid results: Completes assay in 30-60 minutes at room temperature.
- Accurate quantitation: Uses proven copper-chelation chemistry.
- Direct equivalent: Replacement of Pierce™ BCA Protein Assay Kit from Thermo Fisher.
- Versatile applications: Suitable for protein quantitation in cell lysates, serum, and other biological fluids.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11115 | 1000 Tests | Price | |
| 11116 | 5000 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 562 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 31, 2025
