Amplite® Rapid Colorimetric Total Protein Thiol Quantitation Assay Kit
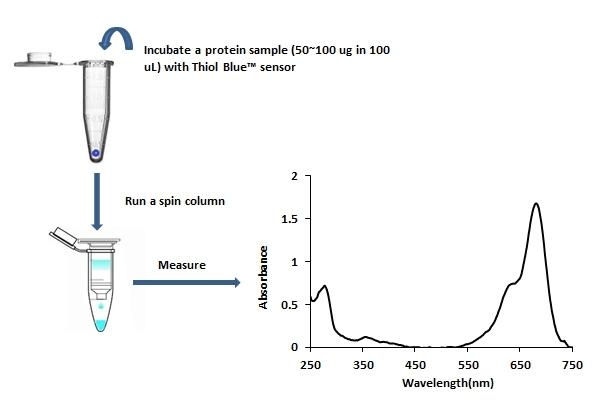
Protein thiols are very important to protein structure, protein function and biological system redox environment. For example, albumin is the most abundant protein in plasma and the free thiol present in the albumin protein are considered as major plasma antioxidants in the body. The change of thiol status in albumin is related to a lot of diseases and disorders, such as kidney disease and Parkinson's disease. Although there are a few reagents or assay kits available for quantitating the total thiol content in biological systems, a key challenge is to have a rapid and accurate method to quantify the amount of free thiol group in a specific protein. Amplite® Rapid Colorimetric Thiol Quantitation Kit provides an accurate method to quantify free thiol group using our proprietary thiol sensor, Thiol Blue™, which has the maximum absorbance at ~680 nm. Thiol Blue™ reacts with the protein samples that contain free thiol groups. The resulted thiol adduct is run through a single spin column to remove the excess Thiol Blue™ sensor, and the absorption spectrum of the purified product is measured. The amount of thiol to protein ratio is calculated from the absorbance ratio of 680 nm and 280 nm. This Amplite® Rapid Colorimetric Thiol Quantitation Kit can be performed in a traditional cuvette, NanoDrop™ Spectrophotometer or a convenient 96-well absorbance plate reader with a UV-transparent plate.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 5529 | 2 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Spectrophotometer | |
| Absorbance | 250 nm~750 nm |
| Recommended plate | Cuvette |
| Absorbance microplate reader | |
| Absorbance | 280 and 680 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 2, 2026
