Cell Navigator® CDy6 Mitosis Imaging Kit
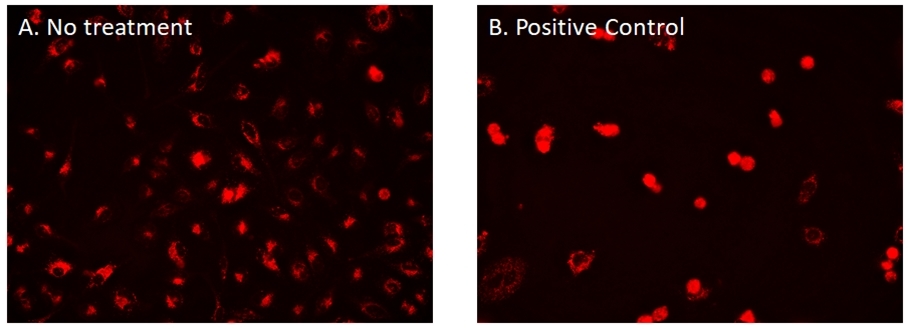
Mitosis is the most defining stage of cell growth. The Cell Navigator® CDy6 Mitosis Imaging Kit is a useful tool for monitoring mitosis by visualizing lysosome dynamics. Long-term real-time visualization of mitosis had been a challenge due to the lack of photostable and low toxicity fluorescent probes. This CDy6 mitosis imaging kit uses a cell permeable lysosome dye, CDy6, which displays high sensitivity in an acidic environment and exhibit bright signal in lysosomes. During mitosis, lysosomes rapidly accumulate towards the nucleus, displaying a sharp increase in signal intensity that can be visualized in real-time. CDy6 does not interfere with the cell cycle and can stand repeated exposure for long-term imaging.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22640 | 100 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microscope | |
| Excitation | Cy3/TRITC filter set |
| Emission | Cy3/TRITC filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 26, 2026
