CL-APC
Cross linked-AlloPhycocyanin
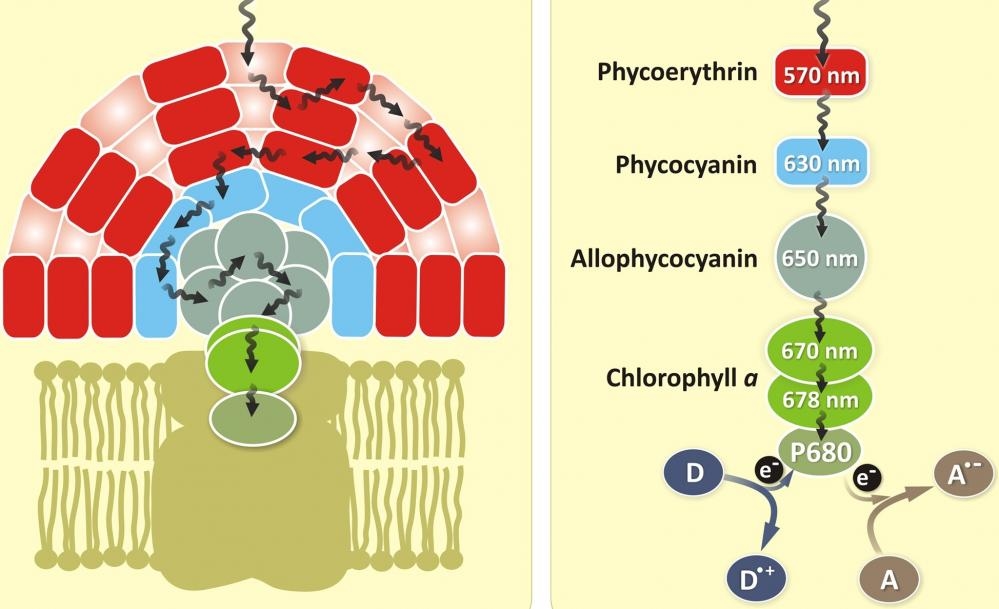
Allophycocyanin (APC) is a phycobiliprotein isolated from Spirulina sp., a blue-green alga. Like other phycobiliproteins, APC is fluorescent, with an extremely high absorptivity and a high quantum efficiency. It is a protein which can be easily linked to antibodies and other proteins by conventional protein cross-linking techniques without altering its spectral characteristics. Allophycocyanin is the least stable among the major phycobiliproteins, susceptible to dissociation at low concentrations including concentrations at which some assays are performed. CL-APC is chemically cross-linked between α and β subunits, and is much more stable than APC. The crosslinked allophycocyanin has improved stability in aqueous solution.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 2549 | 10 mg | Price | |
| 2550 | 50 mg | Price | |
| 2551 | 100 mg | Price | |
| 2552 | 1 mg | Price |
Physical properties
| Molecular weight | ~105000 |
| Solvent | Water |
Spectral properties
| Correction factor (280 nm) | 0.195 |
| Extinction coefficient (cm -1 M -1) | 730000 |
| Excitation (nm) | 651 |
| Emission (nm) | 660 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Refrigerated (2-8 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 26, 2026

