Fura-10™, potassium salt
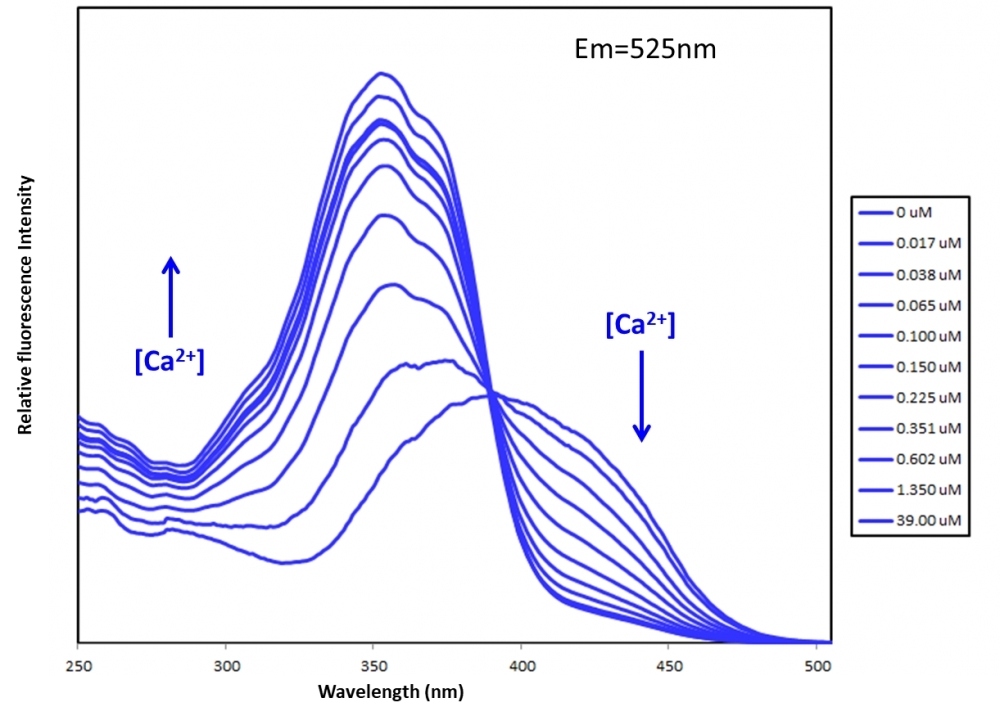
In contrast to single-wavelength indicators such as Fluo-4, the absorption (or fluorescence excitation) maximum of Fura indicators shifts from 380 nm (Fura-2), 415 nm (Fura-8™ and Fura-10™) for the Ca2+-free chelator to about 340 nm (Fura-2), 355 nm (Fura-8™ and Fura-10™) for the Ca2+-bound . The wavelength of maximum fluorescence emission is relatively independent of Ca2+ concentration. The largest dynamic range for Ca2+-dependent fluorescence signals is obtained by using excitation at 340 nm and 380 nm (for Fura-2), 355 nm and 415 nm (for Fura-8™ and Fura-10™) and ratioing the fluorescence intensities detected at ~510 nm (Fura-2), 525 nm (Fura-8™ and Fura-10™). From this ratio, the level of intracellular Ca2+ can be estimated, using dissociation constants (Kd) that are derived from calibration curves. By using the ratio of fluorescence intensities produced by excitation at two wave lengths, factors such as uneven dye distribution and photo bleaching are minimized because they should affect both measurements to the same extent. Calibration solutions should be initially free of heavy metal ions such as manganese, which may affect both its fluorescence and its affinity for calcium.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21110 | 5x50 ug | Price | |
| 21111 | 1 mg | Price |
Physical properties
| Dissociation constant (Kd, nM) | 260 |
| Molecular weight | 1024.27 |
| Solvent | Water |
Spectral properties
| Excitation (nm) | 354 |
| Emission (nm) | 524 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 25, 2026

