Fura-2, AM
CAS 108964-32-5
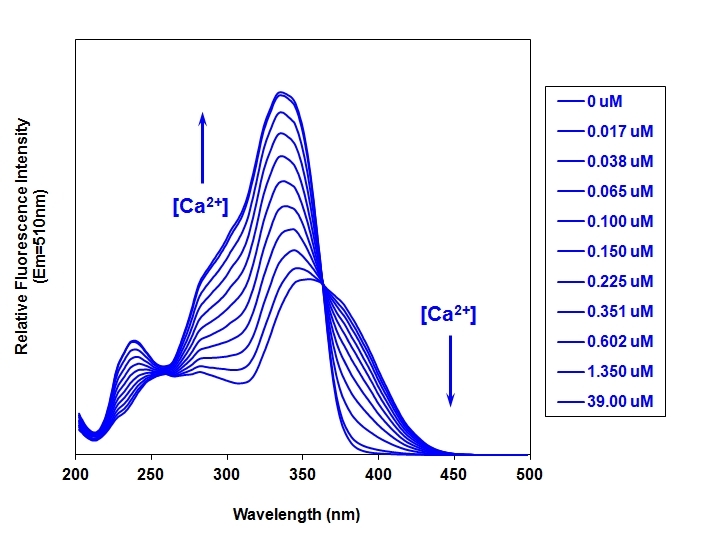
Fura-2 AM is a UV-excitable ratiometric calcium indicator that shifts excitation wavelength upon calcium binding, enabling quantitative measurement of intracellular calcium concentrations independent of dye loading and cell thickness.
- Ratiometric Calcium Measurement: Excitation shift from 380 nm (free) to 340 nm (Ca²⁺-bound)
- Quantitative [Ca²⁺] Determination: Kd = 145 nM, independent of dye concentration or cell thickness
- UV-Excitable Design: Emission at 510 nm allows multicolor experiments with visible dyes
- Calibration-Friendly: Rmax/Rmin determination enables absolute calcium quantification

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21020 | 1 mg | Price | |
| 21022 | 50 mg | Price |
Physical properties
| Dissociation constant (Kd, nM) | 145 |
| Molecular weight | 1001.86 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 336 |
| Emission (nm) | 505 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
| CAS | 108964-32-5 |
Instrument settings
| Fluorescence microscope | |
| Excitation | Fura 2 filter set |
| Emission | Fura 2 filter set |
| Recommended plate | Black wall/clear bottom |
| Fluorescence microplate reader | |
| Excitation | 340, 380 |
| Emission | 510 |
| Cutoff | 475 |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode/Programmable liquid handling |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on October 10, 2024

