Portelite™ Rapid Fluorimetric Endotoxin Detection Kit
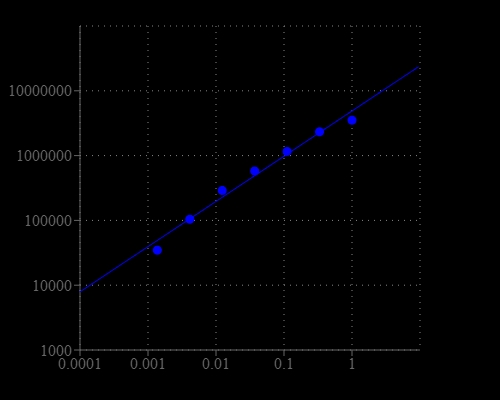
Lipopolysaccharide (LPS), also known as endotoxin, is the major component of the outer membranes of Gram-negative bacteria. LPS is a potent stimulator of the vertebrate innate immune system and can cause fever, septic shock and eventually death. It is also recognized as a biomarker for the detection of bacterial pathogen invasion, and responsible for the development of inflammatory response and endotoxic shock in extreme cases. Detection of LPS in biological materials, such as protein, peptide or antibody sample, is a critical task for biomanufacturing and bioprocessing. Portelite™ Rapid Fluorimetric Endotoxin Detection Kit uses Endotoxin Green™, a sensitive fluorogenic substrate. Endotoxin Green™ can be hydrolyzed in the presence of endotoxins and the Limulus Amebocyte Lysate (LAL), an extract of blood cells from a horseshoe crab, to generate strong green fluorescence. The endotoxin activity is proportional to the fluorescence intensity resulted from the hydrolysis of Endotoxin Green™. The kit is optimized for Cytocite™ and Qubit™ fluorometers, and can detect a broad range of endotoxin (from 1 EU/ml to 0.002EU/ml) present in the sample.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 60008 | 50 Tests | Price | |
| 60009 | 250 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| CytoCite Fluorometer | |
| Excitation | 480 nm |
| Emission | 510-580 nm |
| Instrument specification(s) | 0.2 mL, thin-wall PCR tube |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 17, 2026
