Streptavidin-Xtra™ IF488
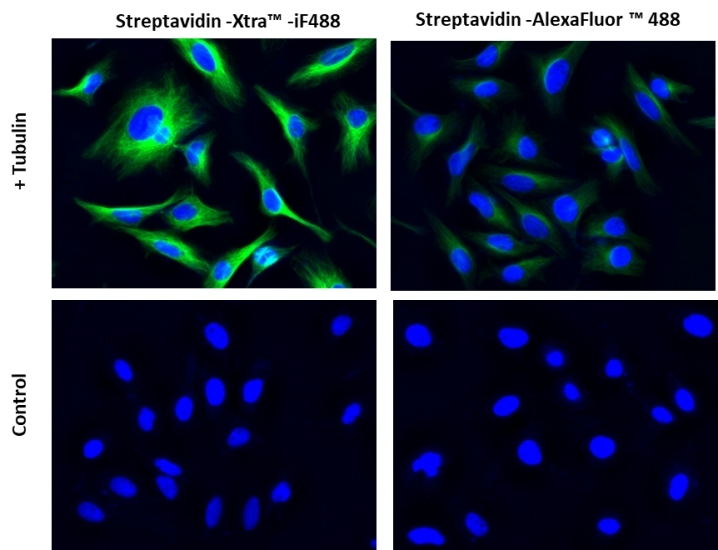
Streptavidin conjugates are widely used in combing with biotin conjugates for detecting a variety of biological targets such as proteins, nucleic acids and other molecules. They are used as an ideal choice for many biological detections such as immunofluorescence microscopy, flow cytometry, western blot and other biological applications since streptavidin has a strong affinity binding biotin which is not affected over a broad range of pH and temperature. AAT Bioquest® offers a variety of streptavidin conjugates labeled with the classic fluorescent dyes (for example: FITC, TRITC, Texas Red®, Cy3®, Cy5® and Cy7®) and also our superior water soluble, photostable iFluor® and mFluor™ dyes. However, the conventional biotin-avidin detection systems are still limited by the limited signal intensity of the existing fluorescent conjugates. The Streptavidin Xtra™ iFluor® conjugates are a new family of super bright streptavidin conjugates with nearly identical excitation and emission properties to Alexa Fluor fluorophores with 3~5 folds signal improvement. It is a set of powerful tools to detect low abundance targets in cell imaging or flow cytometry. iFluor® 488 is one of the most common green fluorescence colors for the FITC channel imaging.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 46000 | 100 ug | Price | |
| 46001 | 1 mg | Price |
Spectral properties
| Correction factor (260 nm) | 0.21 |
| Correction factor (280 nm) | 0.11 |
| Extinction coefficient (cm -1 M -1) | 75000 1 |
| Excitation (nm) | 491 |
| Emission (nm) | 516 |
| Quantum yield | 0.9 1 |
Storage, safety and handling
| Intended use | Research Use Only (RUO) |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm filter |
| Instrument specification(s) | FITC channel |
| Fluorescence microscope | |
| Excitation | FITC filter set |
| Emission | FITC filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 3, 2026

