Tide Quencher™ 1 azide
TQ1 azide
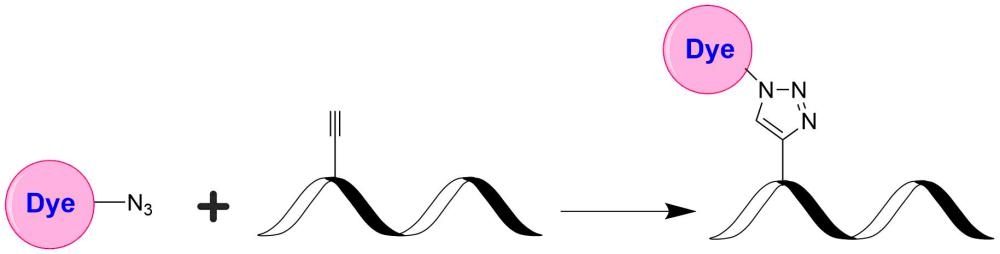
TQ1 is designed to be a superior quencher alternative to DABCYL. Compared to DABCYL, TQ1 have (a). much stronger absorption; (b). much higher quenching efficiency; and (c). versatile reactive forms with desired solubility for labeling oligonucleotides and peptides. This TQ1 product is reactive to alkynes, and useful for click chemistry.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 2188 | 5 mg | Price |
Physical properties
| Molecular weight | 358.42 |
| Solvent | DMSO |
Spectral properties
| Absorbance (nm) | 492 |
| Correction factor (260 nm) | 0.147 |
| Correction factor (280 nm) | 0.194 |
| Extinction coefficient (cm -1 M -1) | 20000 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 22, 2026

