Fluorescent Proteins
GFP was the first fluorescent protein to be identified, and commonly used as a fluorescent tag in biomedical research. GFP originated from the jellyfish Aequorea victoria, and is a roughly 27 kDa protein that emits green light when illuminated with blue or UV light. Many GFP derivatives exist, with varying emission and excitation spectra, which allow for different combinations of green, yellow, cyan and red fluorescent fusion proteins to be visualized in tandem. Each GFP variant also has differing functional properties, is highly stable, and can operate when added to either end of the target protein. Commonly, GFP has been used for determining whether a particular promoter was activated without going through the laborious process of measuring mRNA levels. As GFP can be used in live cells, it is particularly useful for observing a protein as it performs its role within the cell. Recently, novel GFPs have been developed that are derived from other organisms. Though similar to GFP, these proteins diverge widely on sequence-level.
One GFP variant includes TurboGFP, a bright dimeric GFP from the copepod Pontellina plumata. TurboGFP is fast maturing at a wide range of temperatures, has high pH-stability and photostability. Another variant, mNeonGreen, is a monomeric green/yellow fluorescence protein derived from the lancelet Branchiostoma lanceolatum. mNeonGreen has been noted to be up to 3 times brighter than traditional GFP, and also has a fast maturation rate. mNeonGreen has also been reported to act as an acceptor for cyan fluorescent proteins in fluorescence resonance energy transfer (FRET) applications. Both of these variants are evolutionarily distant from the traditional jellyfish-derived fluorescent proteins and share only about a 20% sequence identity with other GFP variants. For this reason, most anti-GFP antibodies do not bind to either of these GFP variants.
Red Fluorescent Proteins (RFPs)
RFPs may also be used, and variants will emit an orange, red, or far-red fluorescence. The first RFP that became commercially available was derived from Discosoma sp. sea anemones, known as DsRed. After DsRed, additional RFPs were identified in other anthozoans (i.e. anemones and corals), but these proteins were mostly tetramers and have not been currently optimized for use in research.
More recently, monomeric RFP derivatives with better fluorescent performance, in terms of brightness, photostability, and higher maturation efficiency, have been created. The RFP variants are powerful tools in research, and include but are not limited to mCherry, mOrange, mRaspberry, mPlum (the “mFruits”), mKO, mRFP, mRFPruby, mRuby, tagRFP, mKate2. DsRed was the first RFP, developed in 1999, but holds some inherent functional problems and is not commonly used in research. DsRed has an incredibly slow maturation time, up to 24 hours or more, and has low photostability making it unusable for shorter experiments. The tetrameric form of DsRed may compromise the function of proteins to which it is attached.
mRFP (also, mRFP1) was the first monomeric variant of DsRed which has a slightly lower levels of absorption, quantum yield, and photostability. However, the maturation rate of mRFP is around 10 times faster than that of DsRed, which results in a similar effective brightness when expressed in living cells. mCherry is one of the most commonly used RFP variants, and offers broad applicability as a fusion protein in various cell types. Like other mFruit RFPs, mCherry is derived from the dsRed variant mRFP1, though has the highest photostability, fastest maturation rate, and excellent pH resistance compared to other members of the mFruit family. A third RFP variant included mPlum, far-red monomeric derivative of mRFP1, which has been widely beneficial for whole-body imaging applications because the main tissue absorbers, such as water, lipids, and hemoglobin, are nearly transparent at the emission range between 650-900 nm.
One GFP variant includes TurboGFP, a bright dimeric GFP from the copepod Pontellina plumata. TurboGFP is fast maturing at a wide range of temperatures, has high pH-stability and photostability. Another variant, mNeonGreen, is a monomeric green/yellow fluorescence protein derived from the lancelet Branchiostoma lanceolatum. mNeonGreen has been noted to be up to 3 times brighter than traditional GFP, and also has a fast maturation rate. mNeonGreen has also been reported to act as an acceptor for cyan fluorescent proteins in fluorescence resonance energy transfer (FRET) applications. Both of these variants are evolutionarily distant from the traditional jellyfish-derived fluorescent proteins and share only about a 20% sequence identity with other GFP variants. For this reason, most anti-GFP antibodies do not bind to either of these GFP variants.
Table 1. Anti-GFP antibodies
| Cat# ▲ ▼ | Product Name ▲ ▼ | Unit Size ▲ ▼ |
| V102110 | Purified Rabbit Anti-GFP Antibody *Polyclonal* | 0.1 mg |
| V103735 | Purified Rabbit Anti-tagged fusion proteins in all species GFP Antibody *PAb (476), monoclonal* | 0.1 mg |
Red Fluorescent Proteins (RFPs)
RFPs may also be used, and variants will emit an orange, red, or far-red fluorescence. The first RFP that became commercially available was derived from Discosoma sp. sea anemones, known as DsRed. After DsRed, additional RFPs were identified in other anthozoans (i.e. anemones and corals), but these proteins were mostly tetramers and have not been currently optimized for use in research.

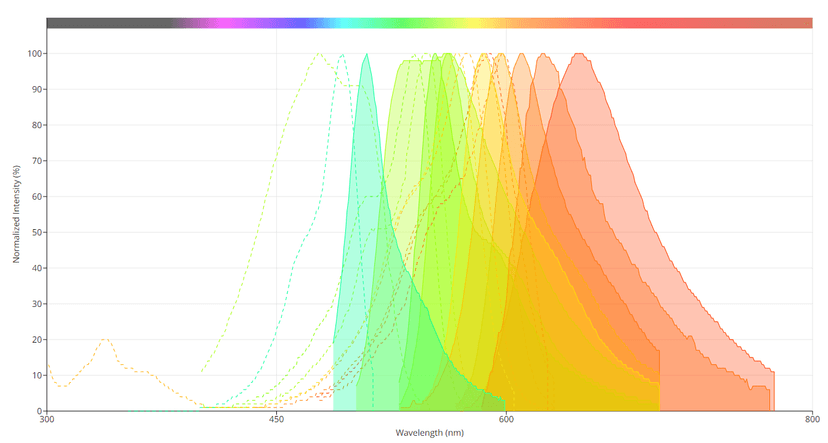
Spectral profiles of several of the 'mFruits' fluorescent proteins. Image from the AAT Bioquest Fluorescence Spectrum Viewer.
mRFP (also, mRFP1) was the first monomeric variant of DsRed which has a slightly lower levels of absorption, quantum yield, and photostability. However, the maturation rate of mRFP is around 10 times faster than that of DsRed, which results in a similar effective brightness when expressed in living cells. mCherry is one of the most commonly used RFP variants, and offers broad applicability as a fusion protein in various cell types. Like other mFruit RFPs, mCherry is derived from the dsRed variant mRFP1, though has the highest photostability, fastest maturation rate, and excellent pH resistance compared to other members of the mFruit family. A third RFP variant included mPlum, far-red monomeric derivative of mRFP1, which has been widely beneficial for whole-body imaging applications because the main tissue absorbers, such as water, lipids, and hemoglobin, are nearly transparent at the emission range between 650-900 nm.