PhosphoWorks™ Fluorimetric Pyrophosphate Assay Kit *Enhanced Selectivity*
Example protocol
AT A GLANCE
Protocol summary
- Prepare PPi Sensor working solution (50 µL)
- Add Pyrophosphate standards and/or test samples (50 µL)
- Incubate at room temperature for 10 to 30 minutes
- Monitor fluorescence intensity at Ex/Em = 370/470 nm (Cutoff = 455 nm)
Important notes
Thaw all the kit components at room temperature before starting the experiment.
PREPARATION OF STOCK SOLUTION
1. PPi Sensor stock solution (200X):
Add 50 µL of DMSO (Component D) into the vial of PPi Sensor (Component B) to make 200X PPi Sensor stock solution. Note: 25 µL of the PPi Sensor stock solution is enough for one 96-well plate.
PREPARATION OF STANDARD SOLUTION
For convenience, use the Serial Dilution Planner: https://www.aatbio.com/tools/serial-dilution/21614
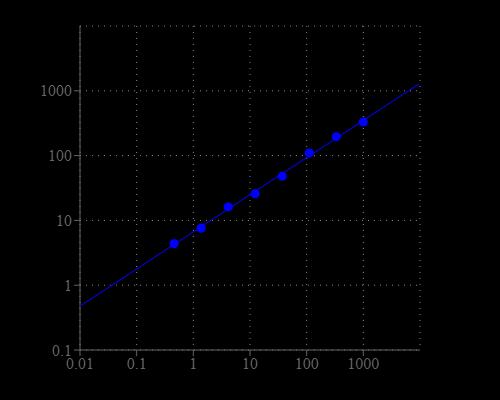
Prepare 1 mM Pyrophosphate standard by adding 10 µL of 50 mM Pyrophosphate Standard (Component C) into 490 µL of Assay Buffer (Component A), or buffer of your choice (preferably 50 mM Hepes buffer, pH 7) to make 1 mM Pyrophosphate standard. Take 1 mM Pyrophosphate standard (PS7) to perform 1:3 serial dilutions to get serially diluted Pyrophosphate standards (PS6-PS1) with Assay Buffer (Component A).
PREPARATION OF WORKING SOLUTION
Add 25 µL of 200X PPi Sensor stock solution to 5 mL of Assay Buffer (Component A), and mix them well. Note: Due to the high sensitivity of this assay to PPi, it is important to use PPi-free labware and reagents. Note: DTT (1 uM) will increase the background, MgCl2 ≥ 2mM decrease the response.
SAMPLE EXPERIMENTAL PROTOCOL
Table 1. Layout of pyrophosphate standards and test samples in a solid black 96-well microplate. PS = Pyrophosphate Standard, BL = Blank Control, TS = Test Sample.
| BL | BL | TS | TS |
| PS1 | PS1 | ... | ... |
| PS2 | PS2 | ... | ... |
| PS3 | PS3 | ||
| PS4 | PS4 | ||
| PS5 | PS5 | ||
| PS6 | PS6 | ||
| PS7 | PS7 |
Table 2. Reagent composition for each well
| Well | Volume | Reagent |
| PS1-PS7 | 50 µL | Serial Dilution (1 to 1000 µM) |
| BL | 50 µL | Assay Buffer (Component A) |
| TS | 50 µL | Sample |
- Prepare Pyrophosphate standards (PS), blank controls (BL), and test samples (TS) according to the layout provided in Tables 1 and 2. For a 384-well plate, use 25 µL of reagent per well instead of 50 µL.
- Add 50 µL of PPi Sensor working solution to each well of Pyrophosphate standard, blank control, and test samples to make the total assay volume of 100 µL/well. For a 384-well plate, add 25 µL of PPi Sensor working solution into each well instead, for a total volume of 50 µL/well.
- Incubate the reaction at room temperature for 10 - 30 minutes, protected from light.
- Monitor the fluorescence increase with a fluorescence plate reader at Ex/Em = 370/470 nm (Cutoff = 455 nm)
Citations
Authors: He, Chunlin and Li, Chuang and Liu, Yao and Chen, Tao-Tao and Li, Chunxiuli and Chu, Xiao and Liu, Shuxin and Wang, Lidong and Zhang, Yong and Ouyang, Songying and others,
Journal: Nature Communications (2025): 1--15
Authors: Cheng, Yuxuan and Ru, Jing and Feng, Chaobo and Liu, Xiaohao and Zeng, Hua and Tan, Shuo and Chen, Xi and Chen, Feng and Lu, Bing-Qiang
Journal: ACS Omega (2024)
Authors: Yang, Shuai and Jin, Shouheng and Xian, Huifang and Zhao, Zhiyao and Wang, Liqiu and Wu, Yaoxing and Zhou, Liang and Li, Mengqiu and Cui, Jun
Journal: Molecular Cell (2023)
Authors: Sun, Fan and Cao, Xueqiang and Yu, Dianzhen and Hu, Dongqiang and Yan, Zheng and Fan, Yingying and Wang, Cheng and Wu, Aibo
Journal: Molecular Plant-Microbe Interactions (2022): 416--427
Authors: Ghavami, Maryam and Merino, Emilio F and Yao, Zhong-Ke and Elahi, Rubayet and Simpson, Morgan E and Fern{\'a}ndez-Murga, Maria L and Butler, Joshua H and Casasanta, Michael A and Krai, Priscilla M and Totrov, Maxim M and others,
Journal: ACS infectious diseases (2017): 549--559