7 Dye qPCR Calibration Plate
Optimized for ABI7500 Fast 96-Well
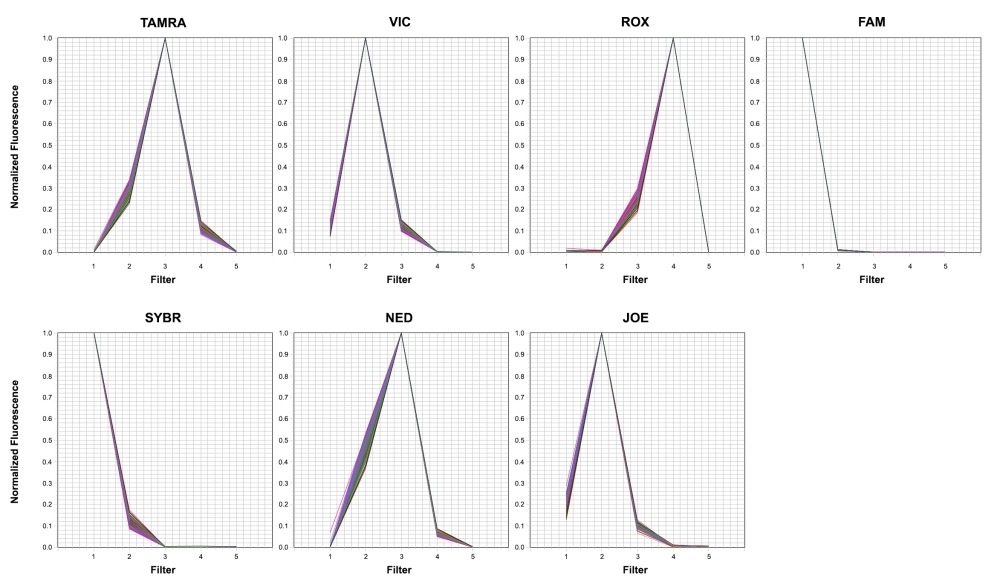
7 Dye qPCR Calibration Plate contains seven spectral calibration plates with seven separate dye standards (TAMRA, SYBR, FAM, JOE, NED, ROX, and VIC). It can be used to maintain your 7500 Real-Time PCR system with Fast 96-well block. For most of qPCR instruments, the necessary calibrations should be run at least every six months. This calibration plate is ready to use without any additional preparation steps required. The qPCR calibration plate might significantly improve qPCR results with multiplexing by more accurately representing fluorescent spectra used in your real-time experiments. Please refer to your instrument's guide for the detailed calibration operation.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 67020 | 1 Set | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 10, 2026
