Amplite® Colorimetric Aspartate Aminotransferase (AST) Assay Kit
The Amplite® Colorimetric Aspartate Aminotransferase Assay Kit offers a convenient and sensitive method for measuring AST activity in biological samples.
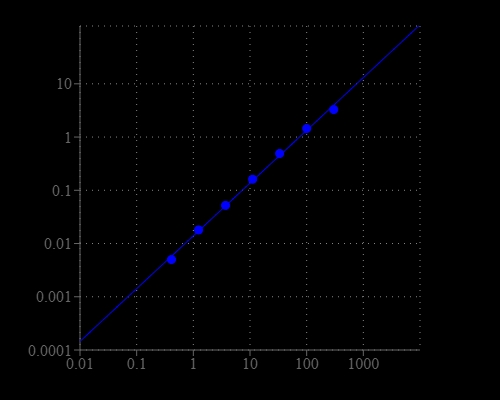
- Sensitive detection: Forms a stable blue product for low AST detection.
- Low detection limit: Capable of detecting as low as 2 mU/mL AST.
- Direct equivalent: Replacement of Sigma's AST/GOT Assay Kit.
- Broad application: Detects AST in serum, plasma, and other samples.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 13801 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 570/610 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026
