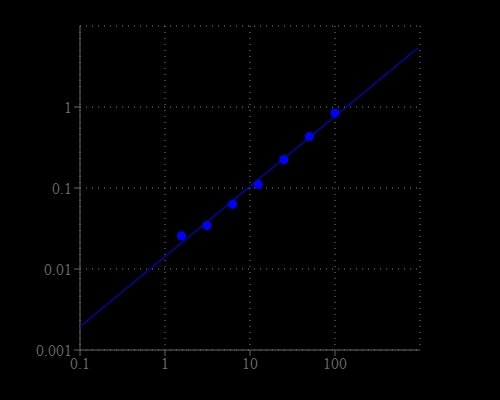
Enterokinase (also called enteropeptidase) is a serine protease produced by cells in the duodenal wall and is a key enzyme in human and animal digestion system. Enterokinase converts trypsinogen into its active form trypsin, resulting in the subsequent activation of pancreatic digestive enzymes. The deficiency of enterokinase results in intestinal digestion impairment. The inhibition of enterokinase may have anti-tumor effects through suppressing proteases involved in carcinogenesis and metastasis. Therefore, highly selective and sensitive detection of enterokinase plays a key role in biochemical applications. Amplite® Colorimetric Enterokinase Activity Assay Kit offers a sensitive assay for quantifying enterokinase activity. After cleavage by enterokinase, the enterokinase substrate can be detected by EK Yellow™ in an absorbance microplate reader at 405 nm.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11410 | 100 Tests | Price |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
| Absorbance microplate reader | |
| Absorbance | 405 nm |
| Recommended plate | Clear bottom |
| Instrument specification(s) | Path check on |
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
