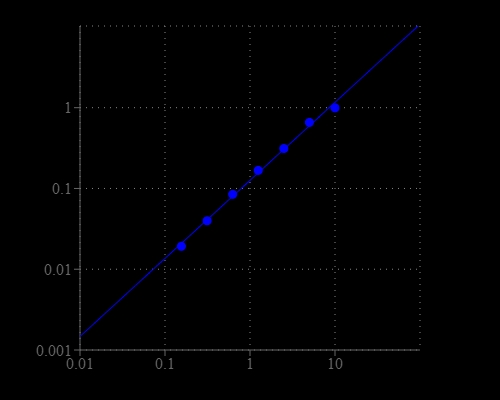
Amplite® Colorimetric Glucose Oxidase Assay Kit
The glucose oxidase is a dimeric protein that catalyzes the oxidation of beta-D-glucose into hydrogen peroxide and D-glucono-1,5-lactone, which is hydrolyzed to gluconic acid. It is widely used for the determination of glucose in body fluids and in removing residual glucose and oxygen from beverages, food and other agricultural products. Furthermore, Glucose oxidase is commonly used in biosensors to detect glucose. The Amplite® Glucose Oxidase Assay Kit provides a quick and sensitive method for the measurement of glucose oxidase in solution. It can be performed in a convenient 96-well or 384-well microtiter plate format and readily adapted to automation without a separation step. The kit uses our Amplite® Red substrate which can be monitored using an absorbance microplate reader at 570 nm.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11299 | 500 Tests | Price |
Spectral properties
| Excitation (nm) | 571 |
| Emission (nm) | 584 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 570 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 4, 2026

