Amplite® Colorimetric Glucose Quantitation Kit
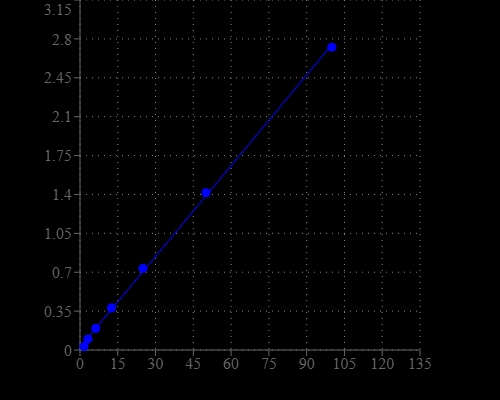
Glucose, a monosaccharide, is the most important carbohydrate in biology. It is a source of energy and metabolic intermediate for cell growth. As one of the main products of photosynthesis, glucose starts cellular respiration in both prokaryotes and eukaryotes. Glucose level is a key diagnostic parameter for many metabolic disorders, e.g., diabetes. This Amplite® Colorimetric Glucose Quantitation Kit provides a quick and sensitive method for the measurement of glucose. It uses glucose oxidase-based enzyme coupled reactions to detect glucose through the production of hydrogen peroxide, which is monitored by our Amplite® Red peroxidase substrate. Amplite® Red peroxidase substrate can be read by an absorbance microplate reader at ~570 nm. The assay is robust, and can be readily adapted for a wide variety of applications that require the measurement of glucose. The assay has very low background since it is run in the red visible range that significantly reduces the interference from biological samples. With the Amplite® Colorimetric Glucose Quantitation Kit, we can detect as little as 3 µM D-glucose.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 40004 | 500 Tests | Price |
Spectral properties
| Excitation (nm) | 571 |
| Emission (nm) | 584 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 570 nm |
| Recommended plate | White or Black wall/Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 13, 2026

