Amplite® Colorimetric Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) Assay Kit
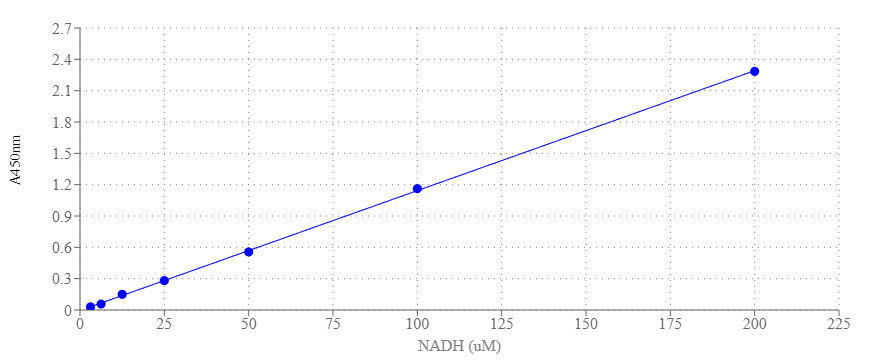
The Amplite® Colorimetric GAPDH Activity Assay Kit is a sensitive and simple tool for monitoring GAPDH activity based on a coupled enzymatic reaction catalyzed by GAPDH. This reaction results in the formation of a colorimetric product with an absorbance at 450nm, which is directly proportional to the enzymatic activity of GAPDH present in cell culture or tissue samples. One unit (U) is the amount of enzyme that catalyzes the reaction of 1 µmol of substrate per minute. GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase) is a multifunctional protein that serves both as a glycolytic enzyme and as a uracil DNA glycosylase. In glycolysis, it catalyzes the conversion of glyceraldehyde-3-phosphate to 1,3-bisphosphate glycerate. GAPDH is also involved in many cellular processes such as apoptosis, membrane trafficking, glucose and iron metabolism, and nuclear translocation. The expression of GAPDH in cells is constitutive, making it a housekeeping gene. Dysregulation of GAPDH activity has been associated with carcinogenesis, abnormal cell growth, and late-onset Alzheimer's disease. Quantification of GAPDH expression levels in different experimental conditions or disease states can provide insights into metabolic changes associated with cellular processes such as proliferation, differentiation, and response to stress.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11321 | 100 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 450 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 3, 2026
