Amplite® Colorimetric Malondialdehyde (MDA) Quantitation Kit
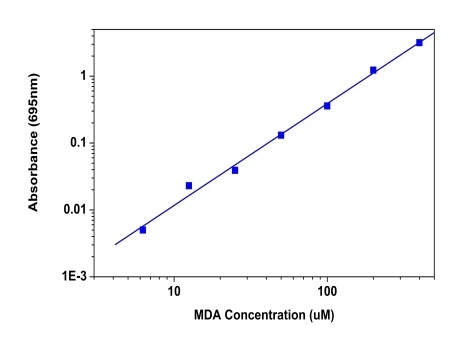
Malondialdehyde (MDA) is natural byproduct of lipid peroxidation and is widely used as a key indicator to determine the oxidative stress and free radical formation. Measurement of MDA has historically relied on the reaction with thiobarbituric acid (TBA) to results in a compound that can be measured colorimetrically at or fluorimetrically. But this assay is not specific to MDA and also takes place under acidic conditions at 90-100 °C. There have been a number of commercial ELISA kits, which makes it more expensive and tedious. The Amplite® Colorimetric Malondialdehyde (MDA) Quantitation Kit offers the most rapid and convenient method to measure MDA without the TBARS heating steps. MDA Blue™ reacts with MDA to generate a blue color product which is measured at 695 nm with absorbance microplate reader. This assay is very fast and specific to MDA with little interference from other aldehydes.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 10070 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 695 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 24, 2026
