Amplite® Colorimetric Total NADP and NADPH Assay Kit
Enhanced Sensitivity
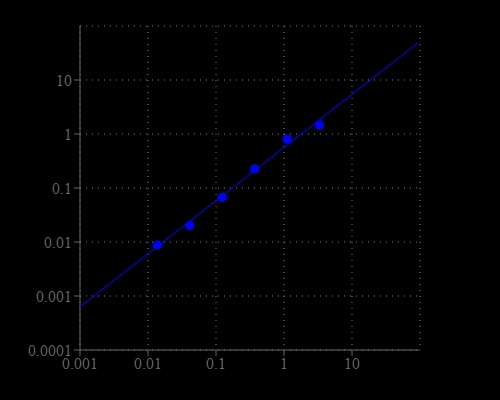
Nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+) are two important cofactors found in cells. NADH is the reduced form of NAD+, and NAD+ is the oxidized form of NADH. It forms NADP with the addition of a phosphate group to the 2' position of the adenyl nucleotide through an ester linkage. NADP is used in anabolic biological reactions, such as fatty acid and nucleic acid synthesis, which require NADPH as a reducing agent. In chloroplasts, NADP is an oxidizing agent important in the preliminary reactions of photosynthesis. The NADPH produced by photosynthesis is then used as reducing power for the biosynthetic reactions in the Calvin cycle of photosynthesis. The traditional NAD/NADH and NADP/NADPH assays are done by monitoring of NADH or NADPH absorption at 340 nm. This method suffers low sensitivity and high interference since the assay is done in the UV range that requires expensive quartz microplate. This Amplite® NADP/NADPH Assay Kit provides a convenient method for sensitive detection of NADP and NADPH. The enzymes in the system specifically recognize NADP/NADPH in an enzyme cycling reaction. There is no need to purify NADP/NADPH from sample mix. The enzyme cycling reaction significantly increases detection sensitivity. Compared to Kit #15260, this kit has higher sensitivity.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 15276 | 400 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 460 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026
