Amplite® Colorimetric Xanthine Assay Kit
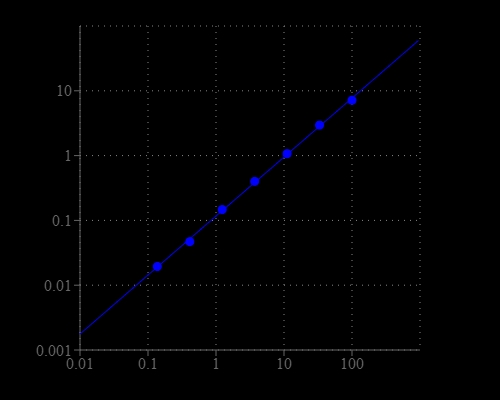
Xanthine is a purine base found in most human body tissues and fluids. A number of stimulants are derived from xanthine, including caffeine, aminophylline, IBMX, paraxanthine, pentoxifylline, theobromine, and theophylline, which can stimulate heart rate, force of contraction, cardiac arrhythmias at high concentrations. Therefore, detection of Xanthine alteration in biological samples is important for disease diagnosis and therapy monitoring. Amplite® Colorimetric Xanthine Assay Kit provides a quick and ultrasensitive method for the measurement of xanthine. It can be performed in a convenient 96-well or 384-well microtiter-plate format. Xanthine is oxidized to uric acid in the presence of xanthine oxidase to release hydrogen peroxide, which can be specifically measured with Amplite® Red by an absorbance microplate reader at 576 nm. With Amplite® Colorimetric Xanthine Assay Kit, as low as 1.2 µM xanthine was detected in a 100 µL reaction volume.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 13842 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 570/610 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 24, 2026
