Amplite® Fluorimetric Alkaline Phosphatase Assay Kit
Green Fluorescence
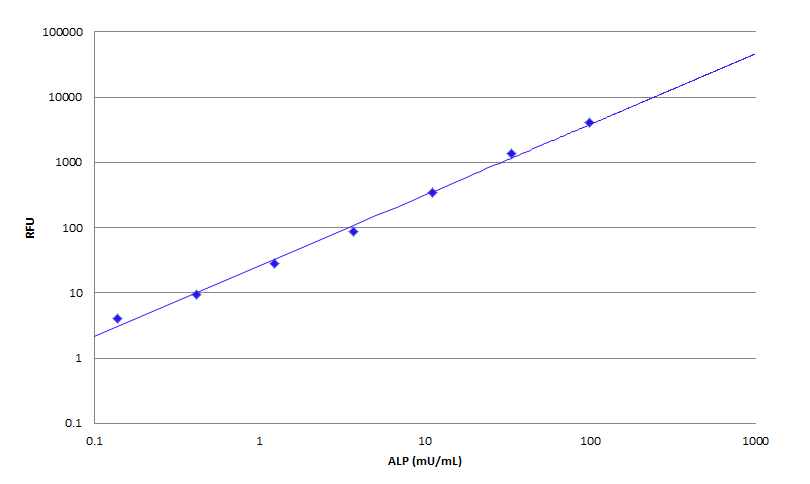
Alkaline phosphatase is a highly sensitive enzyme for ELISA, immuno-histochemical, Northern, Southern and Western blot applications. It is widely used in various biological assays (in particular, immunoassays) and ELISA-based diagnostics. This Amplite® Alkaline Phosphatase Assay Kit uses FDP, a sensitive fluorogenic phosphatase substrate, to quantify alkaline phosphatase activity in solutions, in cell extracts as well as on solid surfaces (such as PVDF membranes). The kit provides all the essential components with our optimized 'mix and read' assay protocol that is compatible with HTS liquid handling instruments.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11953 | 500 Tests | Price |
Spectral properties
| Absorbance (nm) | 487 |
| Correction factor (260 nm) | 0.32 |
| Correction factor (280 nm) | 0.35 |
| Extinction coefficient (cm -1 M -1) | 80000 1 |
| Excitation (nm) | 498 |
| Emission (nm) | 517 |
| Quantum yield | 0.7900 1 , 0.952 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 490 nm |
| Emission | 525 nm |
| Cutoff | 515 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026

