Amplite® Fluorimetric Ascorbic Acid Assay Kit
L-Ascorbic Acid (also called Vitamin C) is a critical metabolite for both plant and animals in cell division, growth and defense. Ascorbate is produced from glucose in the liver of most mammalian species. For humans ascorbate has to be obtained from food to survive, and a lack of sufficient Vitamin C can result in scurvy, and may eventually lead to death. As an antioxidant ascorbate can reduce the risk of developing chronic disease such as cancer and cardiovascular disease. In food industry, ascorbic acid and its sodium, potassium, and calcium salts are commonly used as antioxidant food additives to prevent undesired color and taste. AAT Bioquest's Amplite® Fluorimetric Ascorbic Acid Assay Kit offers a sensitive fluorescent assay for quantifying total ascorbic acid and the ratio of dehydroascorbic acid (DHA) to ascorbic acid in biological samples. It utilizes an enzyme reaction that oxidize ascorbic acid to DHA, which can be detected by Ascorbrite™ Blue with a fluorescence microplate reader. The assay can detect 1uM of total ascorbate in a sample.
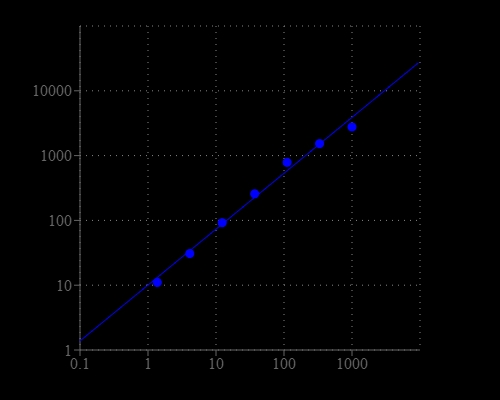

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 13835 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 340 nm |
| Emission | 430 nm |
| Cutoff | 420 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 4, 2026
