Amplite® Fluorimetric Beta-Galactosidase Assay Kit
Green Fluorescence
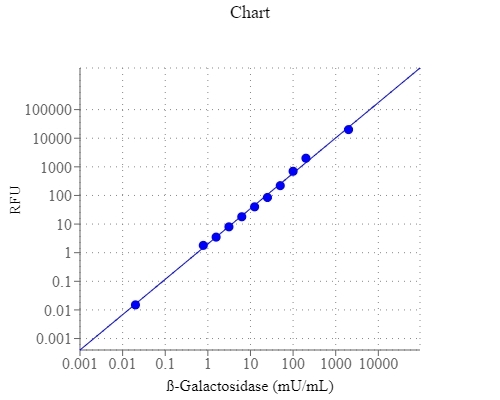
E. coli beta-galactosidase is a 464 kD tetramer. Each unit of beta-galactosidase consists of five domains, the third of which is the active site. It is an essential enzyme in cells. Deficiencies in this enzyme can result in galactosialidosis or Morquio B syndrome. In E. coli, beta-galactosidase is produced by the activation of LacZ operon. Detection of LacZ expression has become routine to the point of detection of as few as 5 copies of β-galactosidase per cell. This kit uses a fluorogenic galactosidase substrate that can sensitively distinguish LacZ+ vs. LacZ-cells. It can be used either for detecting galactosidase conjugates in ELISA type assay systems or for monitoring LacZ gene expression in cells. The galactosidase-cleaved product has an emission spectra that can be detected with most of fluorescence instruments equipped with a FITC filter set.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 12601 | 500 Tests | Price |
Spectral properties
| Absorbance (nm) | 487 |
| Correction factor (260 nm) | 0.32 |
| Correction factor (280 nm) | 0.35 |
| Extinction coefficient (cm -1 M -1) | 80000 1 |
| Excitation (nm) | 498 |
| Emission (nm) | 517 |
| Quantum yield | 0.7900 1 , 0.952 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 490 nm |
| Emission | 525 nm |
| Cutoff | 515 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 5, 2026

