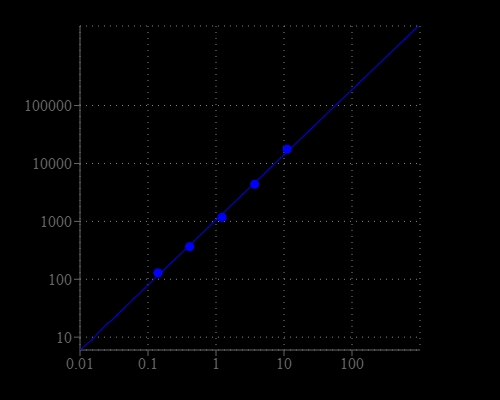
Amplite® Fluorimetric Butyrylcholinesterase Activity Assay Kit
Butyrylcholinesterase (EC 3.1.1.8; BChE), also known as pseudocholinesterase or plasma cholinesterase, is mainly synthesized liver and present in blood. BChE is a nonspecific cholinesterase enzyme and can hydrolyze many different choline esters, serving as the first line of defense against toxic compounds reaching the bloodstream. It has been identified as a biomarker of organophosphate poisoning. Amplite® Fluorimetric Butyrylcholinesterase Activity Assay Kit uses our outstanding Thiolite™ Green to quantify the generation of thiolcholine produced from the hydrolysis of butyrylthiocholine by BChE. The fluorescence intensity of Thiolite Green™ is proportional to the formation of thiolcholine, thus the BChE activity. The assay is convenient, sensitive and can detect as low as 0.1mU/mL in variety of samples.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11407 | 100 Tests | Price |
Storage, safety and handling
| Intended use | Research Use Only (RUO) |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 490 nm |
| Emission | 525 nm |
| Cutoff | 515 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 4, 2026
