Amplite® Fluorimetric DPP4 Activity Assay Kit
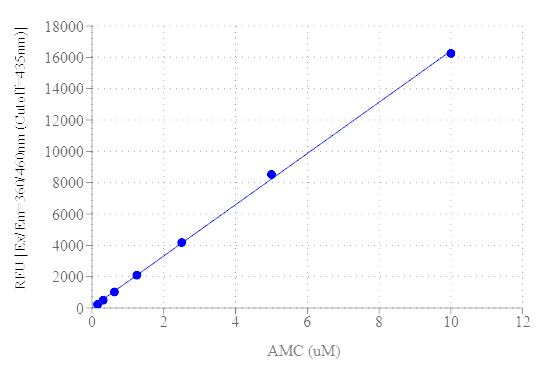
The Amplite® Fluorimetric DPP4 Activity Assay Kit provides a simple, quick, and direct protocol for measuring DPP4 Activity. This assay leverages the DPP4-mediated cleavage of a non-fluorescent substrate, resulting in a fluorescent product with Ex/Em = 360/460 nm, respectively. The fluorescence intensity generated is directly proportional to the DPP4 activity in the sample, making it suitable for detecting DPP4 in various biological matrices such as cellular lysates, tissue extracts, and serum. Additionally, the assay is compatible with high-throughput screening systems. One unit (U) is the amount of enzyme that catalyzes the reaction of 1 µmol of substrate per minute. Dipeptidyl peptidase-4, also known as DPP4, CD26, ADCP2, DPP, is a transmembrane glycoprotein belonging to the prolyl oligopeptidase family. It is a serine exopeptidase that cleaves X-proline and X-Alanine residues from the N-terminal ends of polypeptides. DPP4 is involved in several physiological processes, including glucose metabolism through the regulation of glucagon-like-peptide (GLP-1), immune regulation by acting as a receptor on many immune cells, signal transduction as a transmembrane protein responsive to growth factors and chemokines, and tumor suppression via immune modulation. DPP4 inhibitors are being used as a treatment for type-2 diabetes.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11323 | 100 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 360 nm |
| Emission | 460 nm |
| Cutoff | 435 nm |
| Recommended plate | Solid black or black plate with clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 9, 2026
