Amplite® Fluorimetric DPP4 Inhibitor Screening Kit
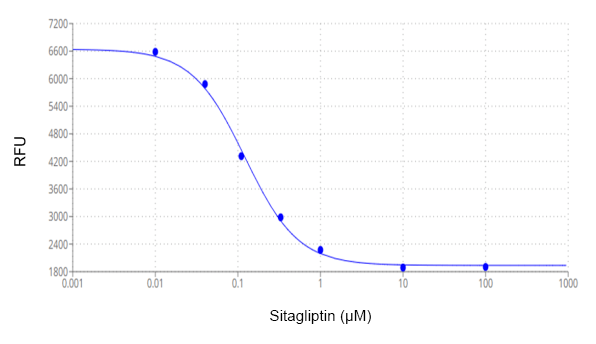
The Amplite® Fluorimetric DPP4 Inhibitor Screening Kit provides an efficient and direct method for evaluating potential DPP4 inhibitors. DPP4 activity is quantified by the enzymatic cleavage of a specific substrate, resulting in a fluorescent product (Ex/Em = 360/460 nm), which correlates directly with the enzymatic activity present. This kit includes Sitagliptin, a well-characterized DPP4 inhibitor used in diabetes treatment, as a reference control, facilitating the assessment of inhibitor efficacy. The assay is optimized for high-throughput screening applications. Dipeptidyl peptidase-4 (DPP4), also referred to as CD26, ADCP2, or DPP, is a transmembrane glycoprotein within the prolyl oligopeptidase family. DPP4 functions as a serine exopeptidase, cleaving N-terminal X-proline and X-alanine residues from polypeptides. It is implicated in numerous physiological processes, including glucose metabolism via regulation of glucagon-like peptide-1 (GLP-1), immune modulation through its role as a receptor on various immune cells, signal transduction as a transmembrane protein responsive to growth factors and chemokines, and tumor suppression via immune system interactions. Thus, DPP4 inhibitors are critical in the therapeutic management of type-2 diabetes.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11324 | 100 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 360 nm |
| Emission | 460 nm |
| Cutoff | 435 nm |
| Recommended plate | Solid black or black plate with clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 4, 2026
