Amplite® Fluorimetric Formaldehyde Quantitation Kit
Green Fluorescence
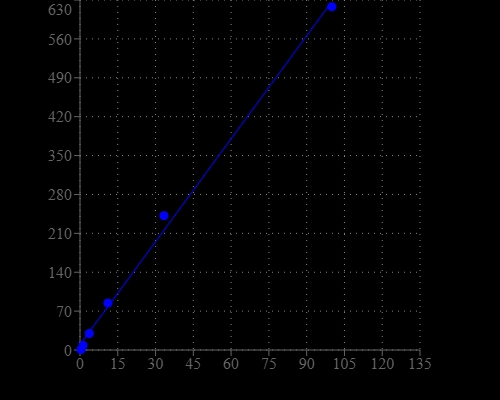
Formaldehyde is a naturally occurring substance. Natural processes in the upper atmosphere may contribute up to 90 percent of the total formaldehyde in the environment. Formaldehyde, as well as its oligomers and hydrates are rarely encountered in living organisms. Methanogenesis proceeds via the equivalent of formaldehyde, but this one-carbon species is masked as a methylene group in methanopterin. Formaldehyde is the primary cause of methanol's toxicity, since methanol is metabolized into toxic formaldehyde by alcohol dehydrogenase. Our Amplite® Fluorimetric Formaldehyde Quantitation Kit are used for quantifying formaldehyde. The kit uses a proprietary fluorogenic dye that generates a strongly fluorescent product upon reacting with formaldehyde. This fluorimetric kit provides a sensitive mix-and-read method to detect formaldehyde. The assay can be performed in a convenient 96-well or 384-well microtiter-plate format and easily adapted to automation without a separation step. Its signal can be easily read with a fluorescence microplate reader.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 10057 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 410 nm |
| Emission | 525 nm |
| Cutoff | 495 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 27, 2026
