Amplite® Fluorimetric Glycerol 3-Phosphate (G3P) Assay Kit
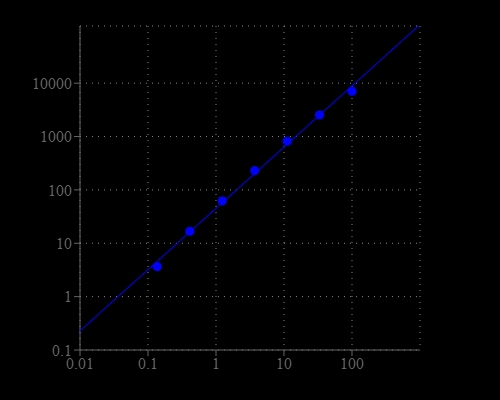
Glycerol 3-Phosphate (G3P) is an important intermediate in the glycolysis metabolic pathway. Animals, fungi, and plants use G3P to produce ATP. It is used to regenerate NAD+ in brain and skeletal muscle cells. G3P has been linked to lipid imbalance diseases such as obesity. Amplite® Glycerol 3-Phosphate Assay Kit provides one of the most sensitive methods for quantifying G3P. The kit uses Amplite® Red substrate to quantify G3P concentration, which is proportional to the production of hydrogen peroxide in the G3P oxidase-mediated enzyme coupling reactions. The kit is an optimized "mix and read" format that is compatible with HTS applications. It detects as little as 41 picomole G3P in 100 µL solution (0.41 µM) as shown in Figure 1. The assay can be performed in a convenient 96-well or 384-well microtiter-plate format without a separation step. Its signal can be easily read with a fluorescence microplate reader.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 13837 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026
