Amplite® Fluorimetric Glycogen Assay Kit
Red Fluorescence
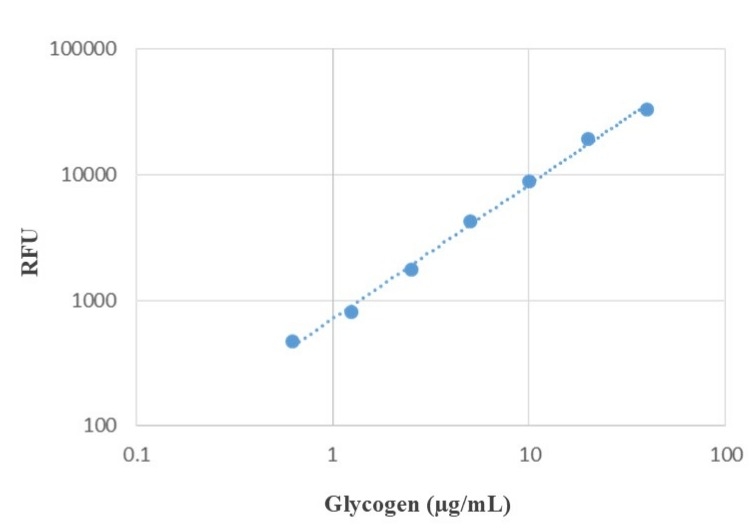
The Amplite® Fluorimetric Glycogen Assay Kit offers a fast, convenient, and sensitive method for quantifying glycogen in biological samples.
- Simple and direct detection: Utilizes an enzymatic hydrolysis step followed by a fluorescence-based reaction to specifically measure glycogen content in samples
- Red fluorescence readout: Generates a bright, stable red fluorescent signal detectable with standard fluorescence microplate readers
- Applications: Ideal for studies in glucose metabolism, diabetes research, and carbohydrate storage disorders
- Novel format: One of the first fluorescence based kits specifically optimized for glycogen quantification

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 40014 | 100 Tests | Price |
Spectral properties
| Excitation (nm) | 571 |
| Emission (nm) | 584 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 19, 2026

