Amplite® Fluorimetric L-Aspartate (Aspartic Acid) Assay Kit
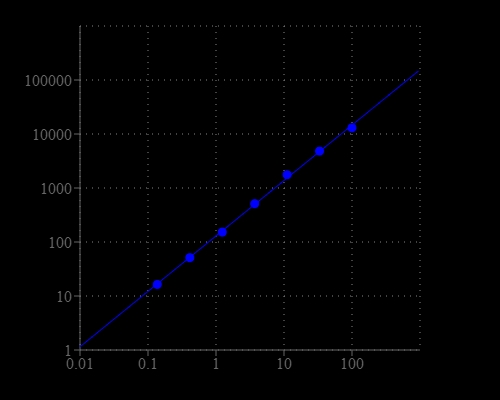
Aspartate (or Aspartic acid) is a negatively charged, polar amino acid. Aspartate is involved in the control point of pyrimidine biosynthesis, in transamination reactions, interconversions with asparagine, in the metabolic pathway leading to AMP, in the urea cycle, and is a precursor to homoserine, threonine, isoleucine, and methionine. It is also involved in the malate aspartate shuttle. Amplite® Fluorimetric Aspartate Assay Kit offers a sensitive fluorescent assay for quantifying aspartate in biological samples. Aspartate is converted to pyruvate that generates hydrogen peroxide through an enzyme coupled reaction. The amount of hydrogen peroxide generated by aspartate is monitored with Amplite® Red substrate for quantifying aspartate by a fluorescence microplate reader.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 13827 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 27, 2026
