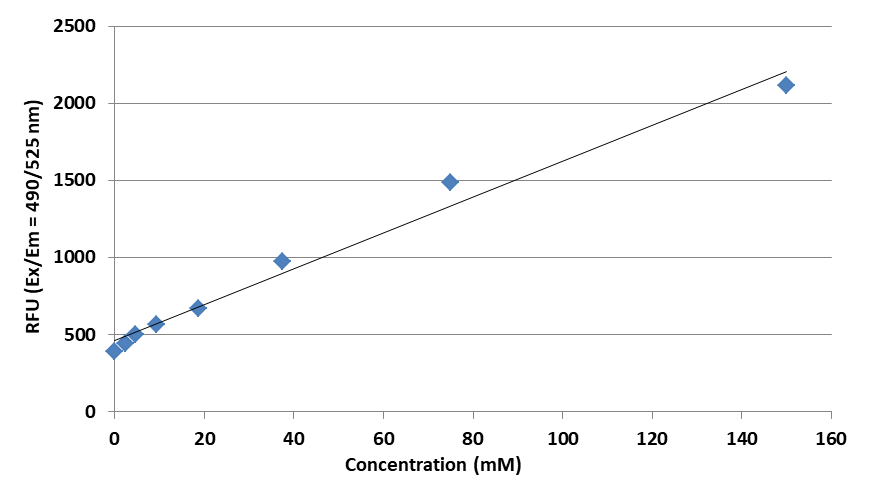
Quantifying lithium ions is important in various scientific fields and industries, including biochemistry, medicine, environmental analysis, and food science, etc. The rapid and accurate determination of lithium ions is particularly important in the battery industry. There are several methods commonly used to quantify lithium ions, including flame photometry, ion-selective electrodes (ISE), atomic absorption spectroscopy, and fluorescence spectrophotometry. Flame photometry and atomic absorption spectroscopy require the inflammation of the samples. They are tedious to use and require expensive and sophisticated instrumentation. Ion-selective electrodes require large volumes of samples and often have low selectivity. Among all the methods, fluorescence spectrophotometry is the most convenient method for quantifying lithium ions. Fluorescence spectrophotometry involves complexing lithium ions with specific reagents and measuring the resulting fluorescence changes. However, there is still lacking a fluorescence-based lithium ion assay kit in the commercial market due to the absence of a robust fluorescence lithium ion indicator. Amplite® Fluorimetric Lithium Ion Quantification Kit uses our new robust lithium-ion indicator dye, Lithiumighty™ 520, which exhibits great fluorescence intensity enhancement upon binding to lithium ions. Lithiumighty ™ 520 is perhaps the most robust lithium-ion indicator with high selectivity. It enables the kit to be useful for the rapid determination of lithium concentrations in a variety of samples compared to the other commercial lithium-ion assays. This microplate-based assay kit requires an extremely small amount of sample. It is particularly suitable for the determination of lithium ion concentration in a microvolume format.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21351 | 100 Tests | Price |
| Excitation (nm) | 491 |
| Emission (nm) | 513 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Fluorescence microplate reader | |
| Excitation | 490 nm |
| Emission | 525 nm |
| Cutoff | 515 nm |
| Recommended plate | Solid black |
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |

