Amplite® Fluorimetric Lysyl Oxidase Assay Kit
Red Fluorescence
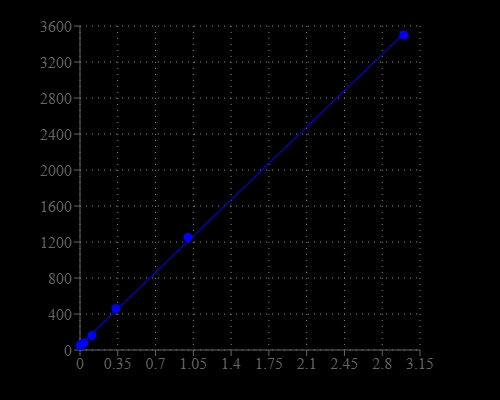
Lysyl oxidase (LOX) is an extracellular enzyme that catalyzes formation of aldehydes from lysine residues in collagen and elastin precursors. These aldehydes are highly reactive, and undergo spontaneous chemical reactions with other lysyl oxidase-derived aldehyde residues, or with unmodified lysine residues. This results in cross-linking collagen and elastin which is essential for stabilization of collagen fibrils and for the integrity and elasticity of mature elastin. Lysyl oxidase has been identified as a possible tumor suppressor. Lysyl oxidase activity in biological samples is traditionally and most reliably assessed by tritium release end-point assays using radiolabeled collagen or elastin substrates involving laborious vacuum distillation of the released tritiated water. This kit offers a sensitive fluorescent assay to measure LOX activity using our proprietary LOX substrate that releases hydrogen peroxide upon LOX oxidation. The amount of hydrogen peroxide released by the LOX oxidation is detected using our Amplite® HRP substrate in the HRP-coupled reactions. This method allows the detection of sub ng/mL lysyl oxidase and is much more sensitive than the currently available fluorimetric assay for this enzyme activity. This method eliminates the interference that occurs in some biological samples and can be readily used to detect lysyl oxidase activity in cell culture experiments. Please note that the kit does not include the lysyl oxidase enzyme.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 15255 | 500 Tests | Price |
Spectral properties
| Excitation (nm) | 571 |
| Emission (nm) | 584 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 30, 2026

