Amplite® Fluorimetric Protein Quantitation Kit
Orange Fluorescence
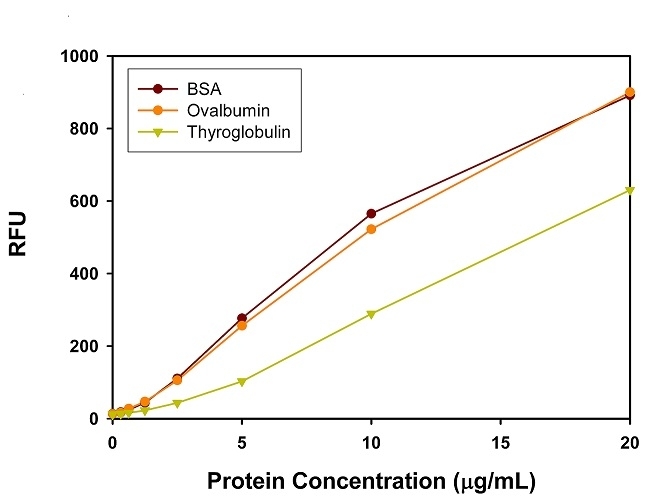
Protein quantification is an essential task in protein purification, electrophoresis, cell biology, molecular biology and other research applications. Biuret, Lowry, BCA and Bradford assays are routinely used for estimating protein concentration. However, these colorimetric assays are less sensitive, and require large sample volume to ensure accuracy. Our Amplite® Fluorimetric Protein Quantitation Kit is significantly more sensitive than existing colorimetric protein measurements, e.g., Bradford and Bicinchoninic acid (BCA) assays. Prolite™ Orange used in the kit is non-fluorescent in aqueous solution, but reacts rapidly with proteins and generates bright fluorescence. The Amplite® Fluorimetric Protein Quantitation Kit provides a simple method for quantifying protein concentration in solutions. As little as 0.1 µg/mL of BSA can be detected. The kit can be performed in a convenient 96-well or 384-well microtiter-plate format. It can be completed within 30 minutes with the fluorescence signal easily monitored. This kit has been used for (1) studying protein/protein interactions; (2) measuring column fractions after affinity chromatography; (3) estimating recovery of membrane proteins from cell extract; and (4) high-throughput screening of fusion proteins.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11105 | 500 tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 485 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 30, 2026
