Amplite® Human Serum Albumin (HSA) Site I Binding Assay Kit
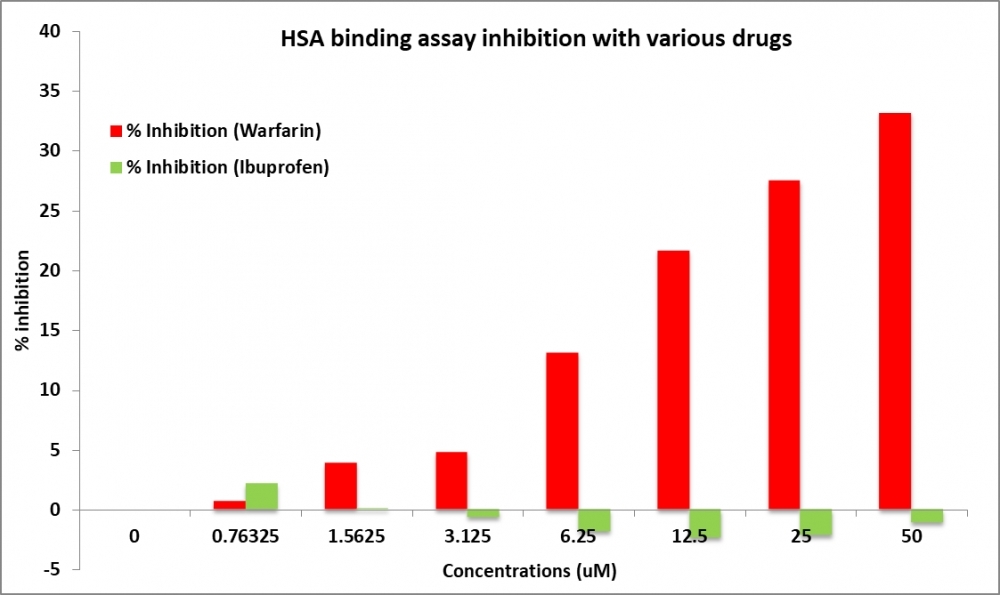
Human serum albumin (HSA) is one of the most important carriers for acidic drugs in human plasma and has been shown to bind a large number of different compounds in a reversible manner. Several different ligand binding sites have been identified for HSA. Among them, Site I has been identified as one of major drug binding sites. Amplite®™ Human Serum Albumin (HSA) Site I Binding Assay Kit is a fluorescence-based high throughput assay to determine the small molecule binding towards HSA. This assay is based on a novel fluorescent probe, HSA Blue™ S1. It has been characterized to bind to the site 1 of HSA with unique spectroscopic and binding properties. HSA Blue™ S1 displays a large fluorescence intensity difference between the protein-bound and protein-unbound state. The competition of small molecules for HSA binding in the presence of HSA Blue™ S1 results in low fluorescence intensities. This assay can be used as a high throughput screen tool to determine total binding to HSA at Site I.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 25400 | 200 Tests | Price |
Spectral properties
| Excitation (nm) | 334 |
| Emission (nm) | 511 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 365 nm |
| Emission | 480 nm |
| Cutoff | 435 nm |
| Recommended plate | Solid black |
| Instrument specification(s) | Top read mode |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 24, 2026

