Amplite® Rapid Colorimetric PE-Maleimide Quantitation Kit
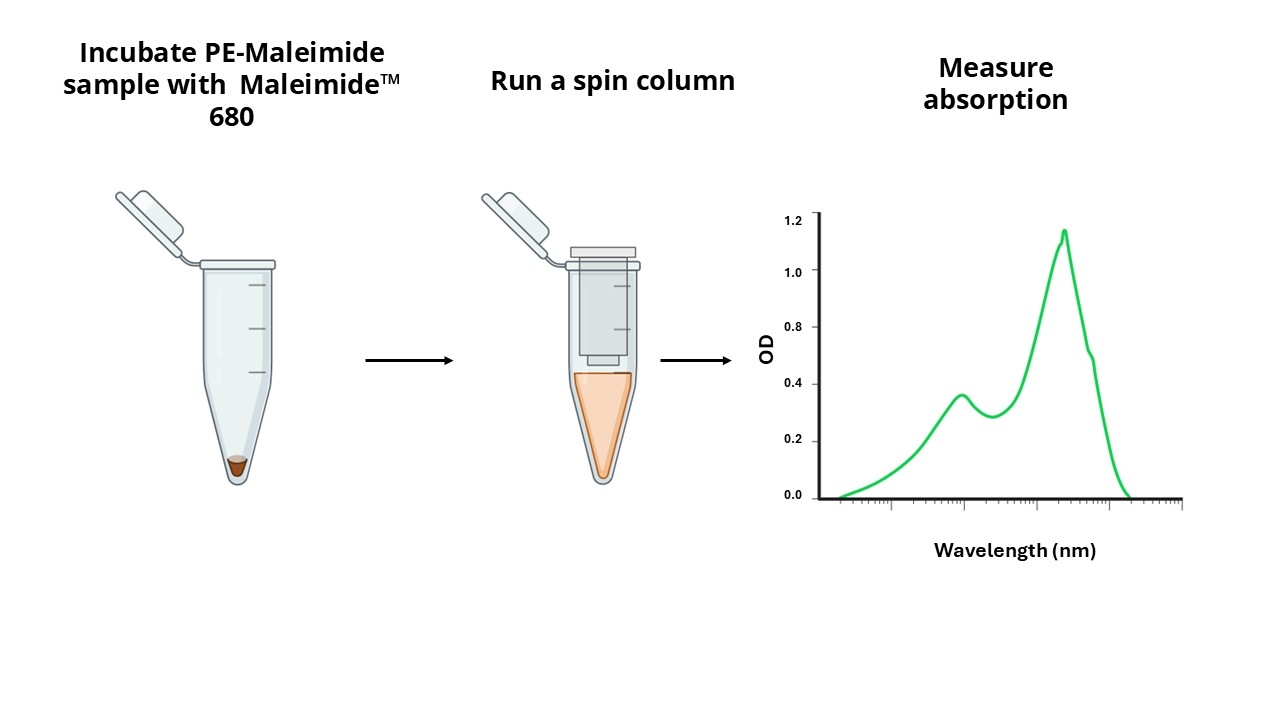
Maleimide-based crosslinking reagents are commonly used for conjugating proteins to other proteins or biomolecules. A significant challenge in maleimide chemistry is the precise quantification of maleimide moieties conjugated to proteins. The Amplite® Rapid Colorimetric PE-Maleimide Quantitation Kit facilitates the quantification of maleimide groups on maleimide-activated PE proteins using a proprietary sensor, Maleimide 680™, which has an absorption peak at ~680 nm. The assay involves the reaction of Maleimide 680™ with the maleimide-activated PE, followed by the separation of the reaction mixture via a spin column to remove the unreacted sensor. Subsequently, the absorption spectrum of the isolated product is measured and the amount of maleimide to PE is determined from the absorbance ratios at 680 nm and 566 nm, with the latter being the peak absorbance of PE. This quantitation kit is compatible with various detection systems, including traditional cuvettes, NanoDrop™ Spectrophotometers, and 96-well absorbance plate readers. It offers a robust and adaptable approach for the rapid quantification of maleimide modifications on PE proteins, addressing a critical need in protein biochemistry.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 5530 | 2 Tests | Price |
Physical properties
| Solvent | DMSO |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 900 nm to 250 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 7, 2026
