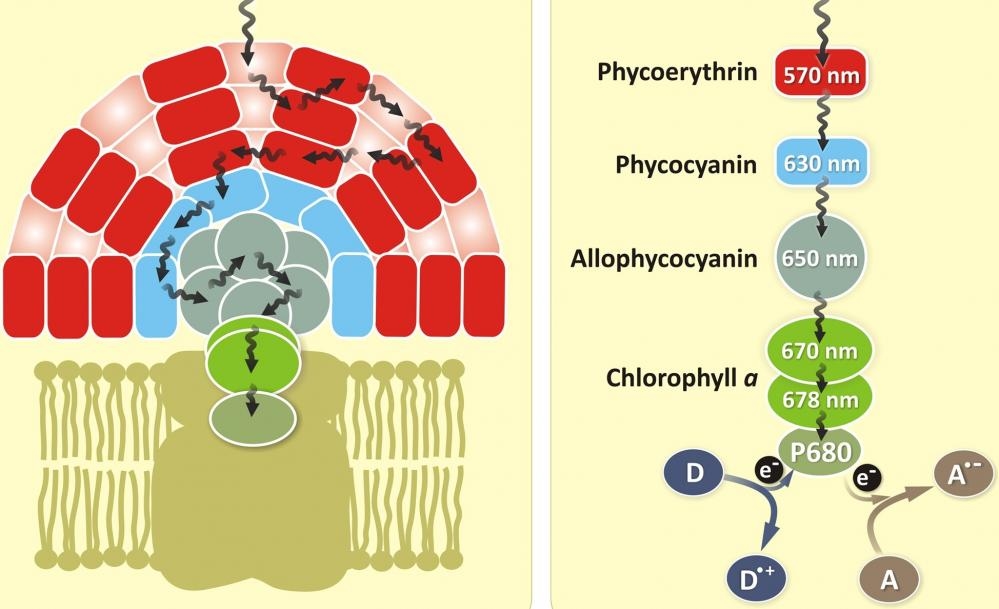
Allophycocyanin (APC) is a phycobiliprotein isolated from Spirulina sp., a blue-green alga. Like other phycobiliproteins, APC is fluorescent, with an extremely high absorptivity and high quantum efficiency. It is a protein which can be easily linked to antibodies and other proteins by conventional protein cross-linking techniques without altering its spectral characteristics.
Allophycocyanin is the least stable among the major phycobiliproteins, susceptible to dissociation at low concentrations including concentrations at which some assays are performed. To address this issue, AAT Bioquest offers CL-APC. This variant is chemically cross-linked between α and β subunits; it is much more stable than APC and has improved stability in aqueous solution.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 2554 | 1 mg | Price | |
| 2555 | 10 mg | Price |
| Molecular weight | ~105000 |
| Solvent | Water |
| Correction factor (280 nm) | 0.195 |
| Extinction coefficient (cm -1 M -1) | 730000 |
| Excitation (nm) | 651 |
| Emission (nm) | 660 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Refrigerated (2-8 °C); Minimize light exposure |
| UNSPSC | 12171501 |
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |

