ApoSight™ Green Caspase 3/7 substrate
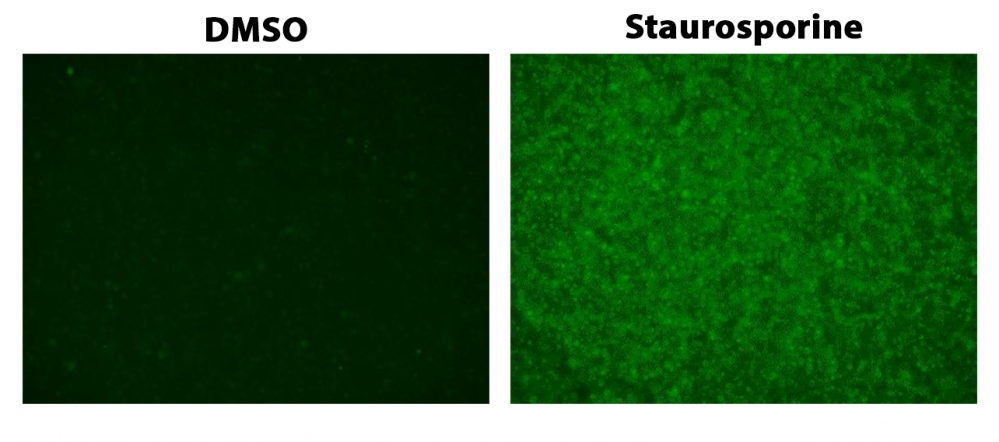
ApoSight™ Green Caspase 3/7 substrate is the first fluorogenic probe for the direct fluorescence imaing of caspase activities in live cells. It consists of three moieties including a). a masked fluorophore, b). a caspase-selective peptide fragment (DEVD), and c). a cell-penetrating moiety. The cell-penetrating moiety carries the probe into live cells. Upon entering live cells, the caspase-selective peptide fragment is cleaved by a caspase to release the masked fluorophore. The intensity of recovered fluorescence is directly related to the activity of the caspase 3/7 to be measured. Compared to the existing caspase assays in live cells, ApoSight™ Green Caspase 3/7 substrate is much more robust, convenient, and accurate. ApoSight™ Green Caspase 3/7 substrate releases a fluorophore that has Ex/Em ~490/520 nm. It does not need a DNA interaction to be fluorescent as reported for NucView reagents. It does not inhibit caspase activity as reported for the FMK peptide probes. Although fluorescent FMK peptide inhibitors of caspases are widely used for detecting caspase activities in live cells, this technology has a few severe limitations: a). FMK caspase inhibitors have high cytotoxicity since FMK peptides bind covalently to active caspases; b). The irreversible covalent binding of FMK peptides to caspases inhibits caspase activities, causing false positive apoptosis; c). FMK assays have extremely high background, and require intensive washings, resulting in extremely low throughput; d). FMK peptides are not stable in aqueous solutions and must be used immediately. ApoSight™ Green Caspase 3/7 substrate can be performed in a convenient 96-well or 384-well microtiter-plate format and easily adapted to automation.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 13290 | 100 Tests | Price |
Physical properties
| Molecular weight | 1290.61 |
| Solvent | DMSO |
Spectral properties
| Extinction coefficient (cm -1 M -1) | 80000 |
| Excitation (nm) | 500 |
| Emission (nm) | 522 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm filter |
| Instrument specification(s) | FITC channel |
| Fluorescence microscope | |
| Excitation | FITC filter set |
| Emission | FITC filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 9, 2026

