Bucculite™ FdU Cu-Free Cell Proliferation Fluorescence Imaging Kit
Red Fluorescence
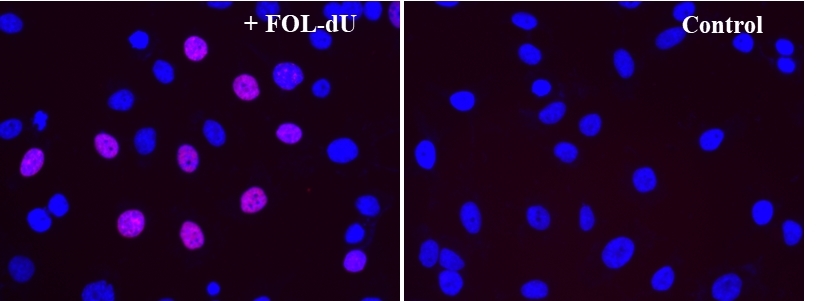
Monitoring cell proliferation is one of the most reliable methods to assess cell viability, cell cycles and genotoxicity. An essential way to detect cell proliferation is to measure DNA synthesis in the presence of thymidine during the S-phase of cells growth. Bucculite™ FdU Cu-Free Cell Proliferation Fluorescence Imaging Kit uses FOL-FdU, an analog of thymidine. FOL-FdU is incorporated into cellular DNA during DNA synthesis. After fixing cells, the incorporated FOL-FdU is labelled with MTA-iFluor® 555 through our Buccutite™ labeling technology. The resulted iFluor® 555-labeled DNA formed in cells is visualized in TRITC Channel. Bucculite™ FdU Cu-Free Cell Proliferation Fluorescence Imaging Kit provides an alternative to anti-BrdU antibody-based assay and EdU click chemistry assay. It is an environment friendly copper-free assay for measuring active DNA synthesis at single-cell level.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22315 | 200 Tests | Price |
Spectral properties
| Correction factor (260 nm) | 0.23 |
| Correction factor (280 nm) | 0.14 |
| Extinction coefficient (cm -1 M -1) | 100000 1 |
| Excitation (nm) | 557 |
| Emission (nm) | 570 |
| Quantum yield | 0.64 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microscope | |
| Excitation | 555 nm |
| Emission | 565 nm |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 9, 2026

