Buccutite™ MTA, maleimide
MTAM
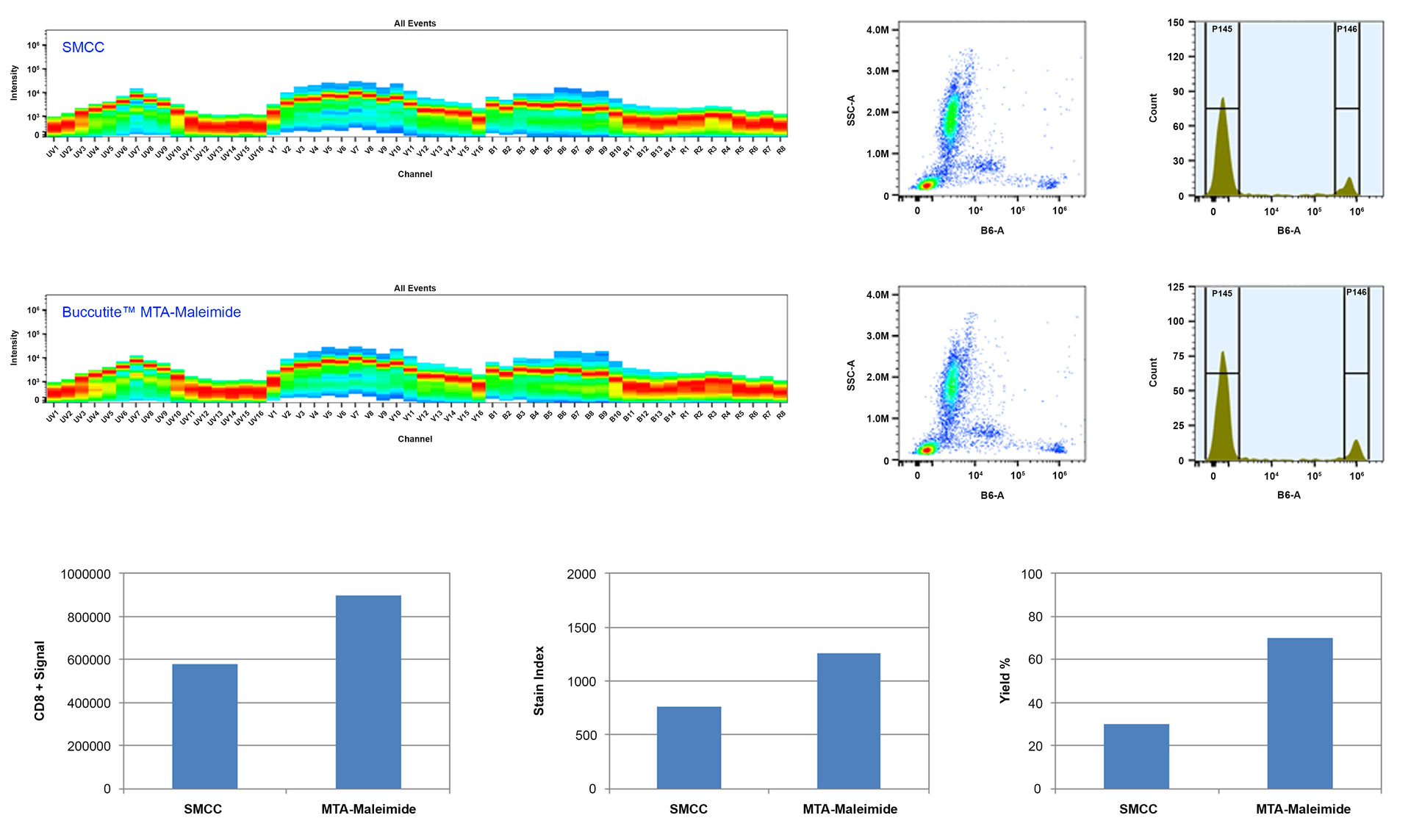
Buccutite™crosslinking technology provides the most convenient and effective crosslinking method to link two biomolecules with a high conjugation yield. The method uses one pair of crosslinkers: Buccutite™ MTA and Buccutite™ FOL. MTA is added to one molecule, while FOL is added to another molecule. The cross-linking reaction is initiated by mixing Molecule-1-Buccutite ™ MTA and Molecule-2-Buccutite ™ FOL under neutral conditions. Many of our customer have requested us to offer the stand-alone Buccutite™ MTA and Buccutite™ FOL reagents to expand the application of Buccutite™crosslinking technology. Buccutite™ MTA maleimide (MTAM) can be used the same way as the widely used SMCC for crosslinking proteins. One end of the MTAM reacts (via maleimide) with thiols (-SH) of cysteine found in the reduced antibodies (by TCEP or DTT). SMCC crosslinking requires high concentration of proteins. In addition, SMCC-modified protein is extremely unstable and often self-reactive since proteins often contain both amine and thiol groups that cause significant amount of homo-crosslinking. Buccutite™ crosslinking reaction occurs under extremely mild and neutral conditions without any catalyst required. It is robust and efficient.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 5358 | 2 umoles | Price |
Physical properties
| Molecular weight | 1151 |
| Solvent | DMSO |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 23, 2026
