Buccutite™ Peroxidase (HRP) Antibody Conjugation Kit
Optimized for Labeling 100 ug Protein
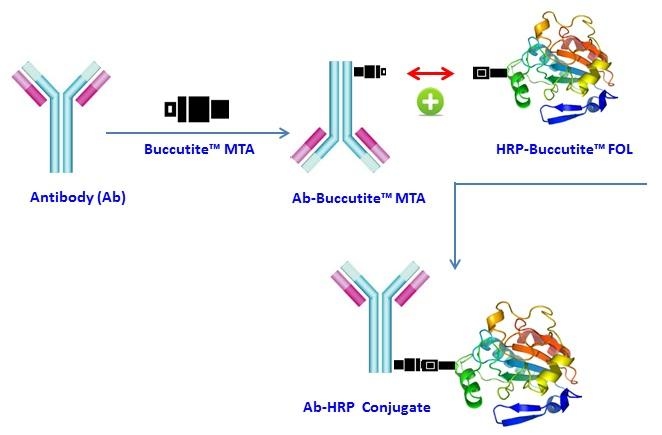
Protein-protein conjugations are commonly performed with a bifunctional linker (such as the commonly used SMCC), having different reactivity on each end for linking two different proteins. One end of the crosslinker reacts (via NHS ester) with amines (-NH2) found in the amino acid lysine and N-terminus, and the other end reacts (via maleimide) with the thiol groups (-SH) found in the amino acid cysteine. However, SMCC-modified protein is extremely unstable and often self-reactive since proteins often contain both amine and thiol groups that cause significant amount of homo-crosslinking. In addition it is quite difficult and tedious to quantify the number of maleimide groups on a protein. Buccutite™ Peroxidase (HRP) Antibody Conjugation Kit is designed for preparing horseradish peroxidase (HRP) conjugates directly from proteins, peptides, and other ligands that contain a free amino group. The HRP provided in our kit has been pre-activated with our proprietary linker Buccutite™ FOL, and can be directly used for conjugation. The Buccutite™ FOL-activated HRP readily reacts with Buccutite™ MTA-containing molecules under extremely mild neutral conditions without any catalyst required. Compared to commonly used SMCC and other similar technologies, our Buccutite™ bioconjugation system is much more robust and easier to use. It enables faster and quantitative conjugation of biomolecules with higher efficiencies and yields.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 5503 | 2 Labelings | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 27, 2026
