Cal-770™, potassium salt
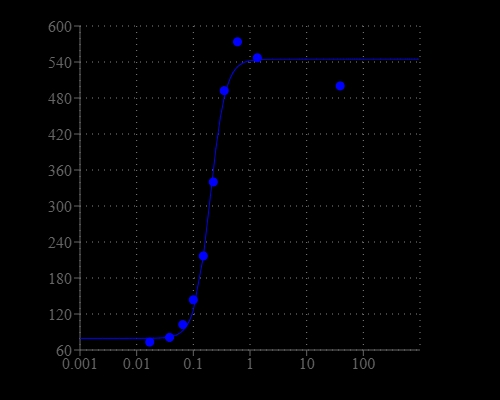
Calcium measurement is critical for numerous biological investigations. Fluorescent probes that show spectral responses upon binding calcium have enabled researchers to investigate changes in intracellular free calcium concentrations by using fluorescence microscopy, flow cytometry, fluorescence spectroscopy and fluorescence microplate readers. Cal-770™ is a near infrared (NIR) calcium indicator with maximum emission at ~775 nm. It is the only fluorescent calcium indicator with excitation and emission longer than 700 nm with a moderate calcium affinity of Kd ~850 nM. Cal-770™ is one of the very few calcium indicators that can be potentially used for in vivo imaging since it has a NIR fluorescence.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 20460 | 10x50 ug | Price |
Physical properties
| Dissociation constant (Kd, nM) | 850 |
| Molecular weight | 1688.58 |
| Solvent | Water |
Spectral properties
| Excitation (nm) | 758 |
| Emission (nm) | 783 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026

