Cal Red™ R525/650 potassium salt
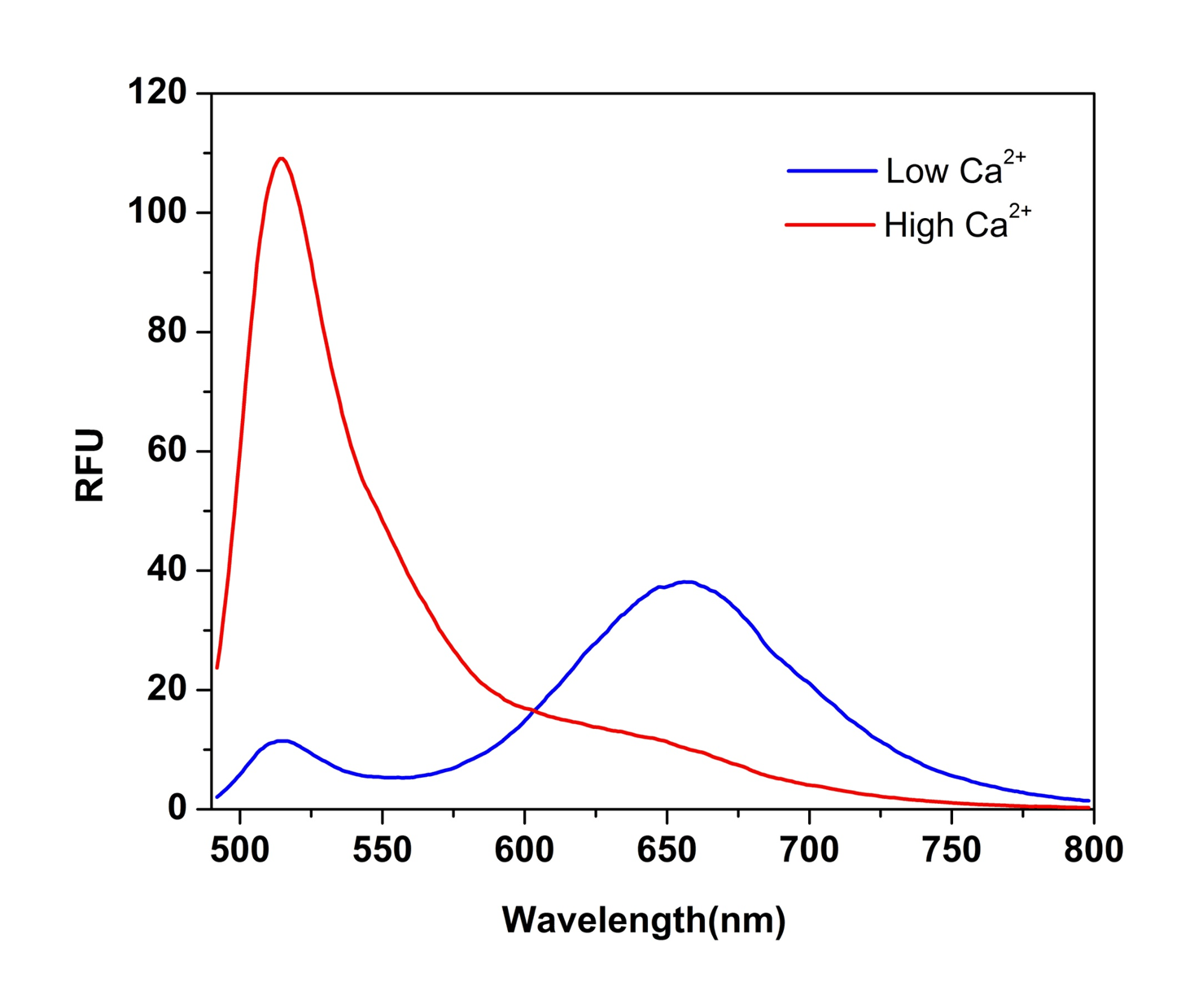
The intracellular calcium flux assay is a widely used method for monitoring the activities of GPCRs and calcium channels. To quantify the intracellular calcium concentration, ratiometric fluorescent calcium indicators are preferred because the ratio is directly related to the calcium concentration and independent of the cell numbers and dye loading concentration. However, the most popular ratiometric calcium indicators (such as Fura-2 and Indo-1) have certain limitations such as lower sensitivity, UV excitation, and not compatible with HTS screening filter set. Cal Red™ R525/650 has been developed as a new 488 nm-excitable ratiometric fluorescence calcium indicator. Cal Red™ R525/650 is well excited at 488 nm with two emissions at 525 nm and 650 nm. Upon binding calcium, the emission signal of Cal Red™ R525/650 is increased at 525 nm and decreased at 650 nm when excited at 488 nm. The excitation and emission wavelength of Cal Red™ R525/650 are compatible with common filter sets with minimal damage to cells, making it a robust tool for evaluating and screening GPCR agonists and antagonists as well as calcium channel targets.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 20588 | 5x50 ug | Price |
Physical properties
| Dissociation constant (Kd, nM) | 330 |
| Molecular weight | ~1000 |
| Solvent | Water |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 23, 2026
