Calcein, AM
UltraPure grade; CAS 148504-34-1
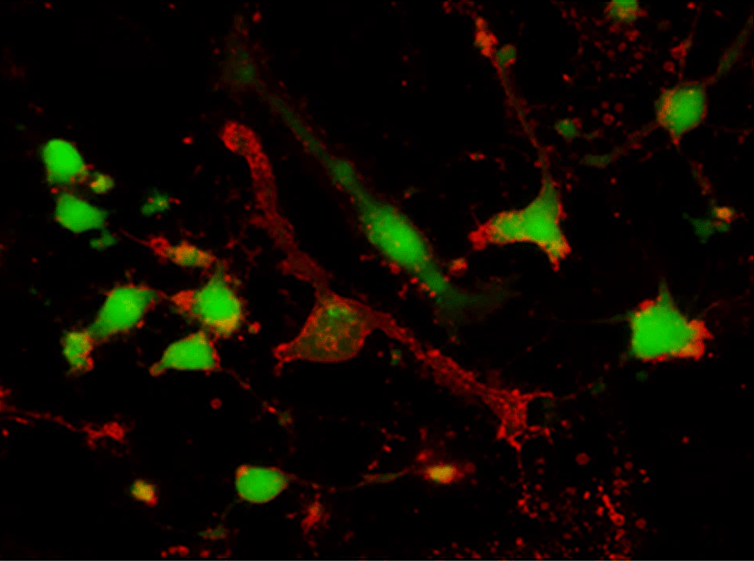
Calcein AM is a cell-permeable, non-fluorescent compound that becomes intensely green fluorescent upon cleavage by intracellular esterases in viable cells, serving as a gold standard for cell viability and cytotoxicity assays.
- Esterase-Activated Fluorescence: Non-fluorescent AM ester cleaved to green fluorescent calcein (Ex/Em = 501/521 nm)
- Viability-Specific Loading: Requires active intracellular esterases and intact membranes
- High Retention in Live Cells: Hydrophilic calcein trapped intracellularly, enhanced with probenecid
- Quantitative Cell Health: Linear correlation between fluorescence intensity and viable cell number

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22003 | 1 mg | Price | |
| 22004 | 20x50 ug | Price |
Physical properties
| Molecular weight | 994.86 |
| Solvent | DMSO |
Spectral properties
| Extinction coefficient (cm -1 M -1) | 81000 |
| Excitation (nm) | 501 |
| Emission (nm) | 521 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
| CAS | 148504-34-1 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm filter |
| Instrument specification(s) | FITC channel |
| Fluorescence microscope | |
| Excitation | FITC filter set |
| Emission | FITC filter set |
| Recommended plate | Black wall/clear bottom |
| Fluorescence microplate reader | |
| Excitation | 490 |
| Emission | 525 |
| Cutoff | 515 |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on September 13, 2024

