Calcein UltraGreen™ AM
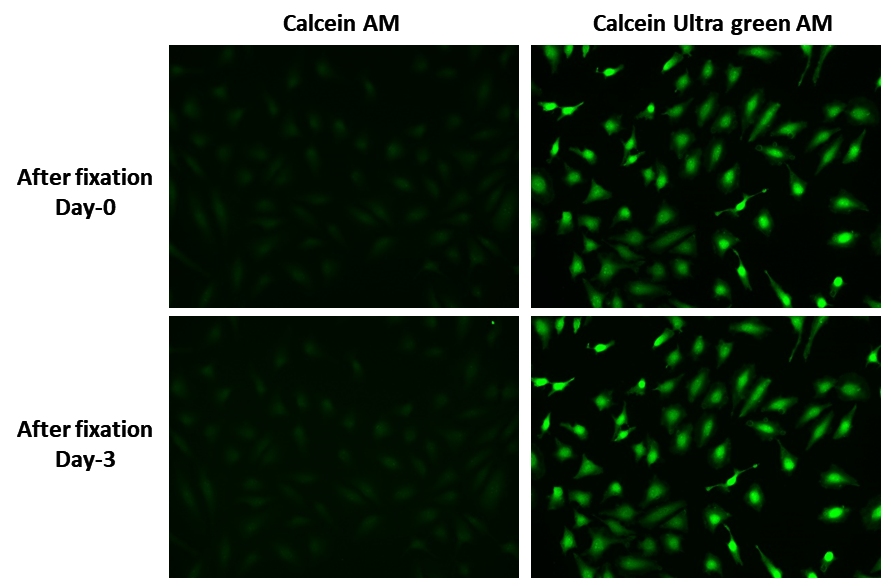
Calcein UltraGreen™ AM readily passes through the cell membrane of viable cells. Upon transporting into live cells cellular esterases cut off the AM groups, the molecule gets trapped inside cells. Compared with Calcein AM, Calcein UltraGreen™ is more suitable fluorescent probe for staining viable cells because of its lower cytotoxicity and longer retention in cells. UltraGreen™ AM does not significantly affect cellular functions such as proliferation or chemotaxis of lymophocyte.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21905 | 10x50 ug | Price |
Physical properties
| Molecular weight | 607.47 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 492 |
| Emission (nm) | 514 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm filter |
| Instrument specification(s) | FITC channel |
| Fluorescence microscope | |
| Excitation | FITC filter set |
| Emission | FITC filter set |
| Recommended plate | Black wall/clear bottom |
| Fluorescence microplate reader | |
| Excitation | 490 |
| Emission | 525 |
| Cutoff | 515 |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 23, 2025

